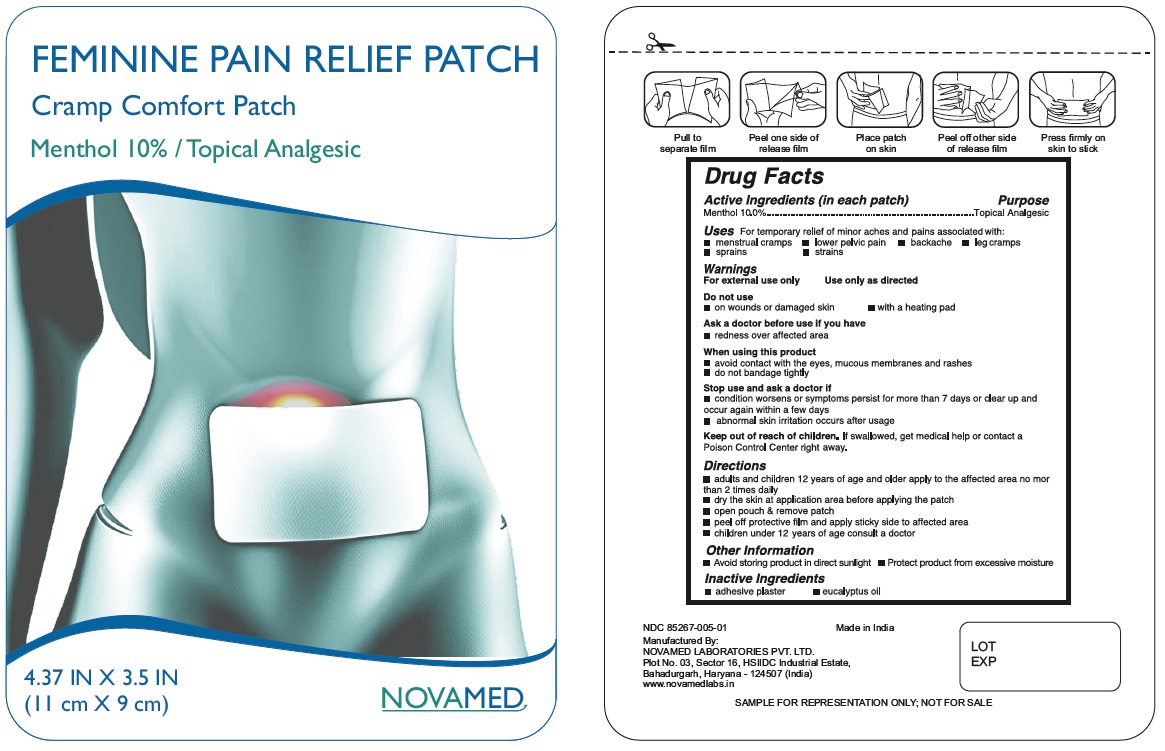
Menthol 10% FEMININE PAIN RELIEF PATCH Drug Facts
FEMININE PAIN RELIEF by
Drug Labeling and Warnings
FEMININE PAIN RELIEF by is a Otc medication manufactured, distributed, or labeled by Novamed Laboratories Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FEMININE PAIN RELIEF- menthol patch
Novamed Laboratories Private Limited
----------
Menthol 10% FEMININE PAIN RELIEF PATCH
Drug Facts
Active Ingredient (in each patch) Purpose
Menthol 10.0%........................................Topical analgesic
Uses
For temporary relief of minor aches and pains associated with:
- menstrual cramps
- lower pelvic pain
- backache
- leg cramps
- sprains
- strains
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days or clear up and occur again within a few days
- abnormal skin irritation occurs after usage
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- adults and children 12 years of age and older apply to the affected area no more than 2 times daily
- dry the skin at application area before applying the patch
- open pouch and remove patch
- peel off protective film and apply sticky side to affected area
- children under 12 years of age consult a doctor
Other Information
- Avoid storing product in direct sunlight.
- Protect product from excessive moisture.
| FEMININE PAIN RELIEF
menthol patch |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Novamed Laboratories Private Limited (766401623) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novamed Laboratories Private Limited | 766401623 | manufacture(85267-005) , analysis(85267-005) , label(85267-005) , pack(85267-005) | |
Revised: 8/2025
Document Id: 3ccc05b3-6210-d6f1-e063-6394a90a54f5
Set id: 0c08bc4f-b99f-40cf-9cd7-06e2e8ad808f
Version: 1
Effective Time: 20250820