ALCAINE- proparacaine hydrochloride solution/ drops
Alcaine by
Drug Labeling and Warnings
Alcaine by is a Prescription medication manufactured, distributed, or labeled by Alcon Laboratories, Inc., Alcon Research, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% is a topical local anesthetic for ophthalmic use. The active ingredient is represented by the structural formula:
Established name:
Proparacaine Hydrochloride
Chemical name:
Benzoic acid, 3-amino-4-propoxy-,2-(diethylamino) ethyl ester, monohydrochloride.
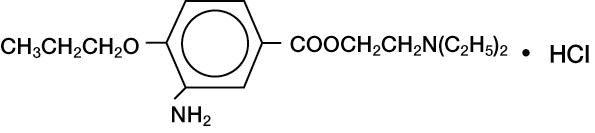
Molecular Weight: 330.85
Each mL contains: Active: proparacaine hydrochloride 5 mg 0.5%. Preservative: benzalkonium chloride (0.01%). Inactives: glycerin and purified water. The pH may be adjusted with hydrochloric acid and/or sodium hydroxide.
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
Carcinogenesis, Mutagenesis, Impairment of Fertility.
Long-term studies in animals have not been performed to evaluate carcinogenic potential, mutagenicity, or possible impairment of fertility in males or females.
Pregnancy: Pregnancy Category C:
Animal reproduction studies have not been conducted with ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5%. It is also not known whether proparacaine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Proparacaine hydrochloride should be administered to a pregnant woman only if clearly needed.
- Nursing Mothers:
-
Pediatric Use:
Safety and effectiveness of proparacaine hydrochloride ophthalmic solution in pediatric patients have been established. Use of proparacaine hydrochloride is supported by evidence from adequate and well-controlled studies in adults and children over the age of twelve, and safety information in neonates and other pediatric patients.
- Geriatric Use:
-
ADVERSE REACTIONS:
Occasional temporary stinging, burning and conjunctival redness may occur with the use of proparacaine. A rare, severe, immediate-type, apparently hyperallergic corneal reaction characterized by acute, intense and diffuse epithelial keratitis, a gray, ground glass appearance, sloughing of large areas of necrotic epithelium, corneal filaments and, sometimes, iritis with descemetitis has been reported.
Allergic contact dermatitis from proparacaine with drying and fissuring of the fingertips has also been reported.
-
DOSAGE AND ADMINISTRATION:
Usual Dosage: Removal of foreign bodies and sutures, and for tonometry: 1 to 2 drops (in single instillations) in each eye before operating.
Short corneal and conjunctival procedures: 1 drop in each eye every 5 to 10 minutes for 5 to 7 doses.
NOTE: ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% should be clear to straw-color. If the solution becomes darker, discard the solution.
-
HOW SUPPLIED:
ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% is supplied in 15 mL DROP-TAINER® dispensers.
NDC 0998-0016-15
Storage:
Bottle must be stored in unit carton to protect contents from light. Store bottles under refrigeration at 2° - 8°C (36° - 46°F).
Rx Only
©2004 Alcon, Inc.
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134 USA
Printed in USA
MedInfo@AlconLabs.com
1-800-757-9195
249039-0609
-
PRINCIPAL DISPLAY PANEL
NDC 0998-0016-15
Alcon®
Alcaine®
(proparacaine hydrochloride ophthalmic solution USP) 0.5%
15 mL Sterile
Rx Only
PRECAUTION: NOT FOR INJECTION. FOR TOPICAL OPHTHALMIC USE ONLY. Do not touch dropper tip to any surface, as this may contaminate the solution.
USUAL DOSAGE: 1 or 2 drops. Read enclosed insert.
Bottles must be stored in unit carton to protect from light.
STORAGE: Store between 2º-8ºC (36º-46ºF).
ALCAINE® (proparacaine hydrochloride ophthalmic solution) should be clear to straw-color. If the solution becomes darker, discard the solution.
INGREDIENTS: Each mL contains: Active: proparacaine hydrochloride 5 mg (0.5%). Preservative: benzalkonium chloride 0.01%. Inactives: glycerin, hydrochloric acid and/or sodium hydroxide (to adjust pH), purified water
pH range 4.0 to 6.0
©2001, 2004, 2009 Alcon, Inc.
1-800-757-9195
MedInfo@AlconLabs.com
Alcon®
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134 USA
Printed in USA
359039-0509
LOT:
EXP.:

NDC 0998-0016-15
Alcon®
Alcaine®
(proparacaine hydrochloride ophthalmic solution USP) 0.5%
Sterile 15 mL
Rx Only
PRECAUTION: NOT FOR INJECTION. FOR TOPICAL OPHTHALMIC USE ONLY. Do not touch dropper tip to any surface as this may contaminate the solution.
USUAL DOSAGE: 1 or 2 drops. Read enclosed insert.
STORAGE: Store between 2º-8ºC (36º-46ºF).
Bottles must be stored in unit carton to protect from light.
ALCAINE® (proparacaine hydrochloride ophthalmic solution) should be clear to straw-color. If the solution becomes darker, discard the solution.
INGREDIENTS: Each mL contains: Active: proparacaine hydrochloride 5 mg (0.5%).
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA Printed in USA
© 2001, 2004 Alcon, Inc.
LOT/EXP.:
H12934-0813

-
INGREDIENTS AND APPEARANCE
ALCAINE
proparacaine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0998-0016 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPARACAINE HYDROCHLORIDE (UNII: U96OL57GOY) (PROPARACAINE - UNII:B4OB0JHI1X) PROPARACAINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0998-0016-15 1 in 1 CARTON 10/19/1973 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA080027 10/19/1973 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research Ltd 007672236 manufacture(0998-0016)
Trademark Results [Alcaine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALCAINE 76218842 2614575 Live/Registered |
ALCON RESEARCH, LTD. 2001-03-02 |
 ALCAINE 73198884 1130668 Dead/Cancelled |
ALCON LABORATORIES, INC. 1978-12-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.