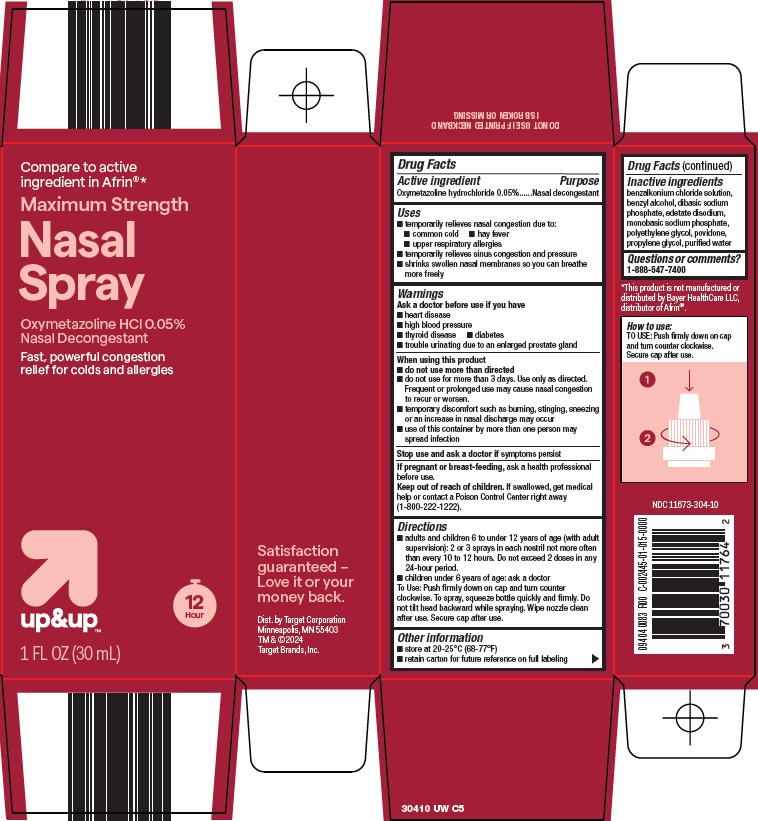
Target Corporation Nasal Spray Drug Facts
Up and up nasal by
Drug Labeling and Warnings
Up and up nasal by is a Otc medication manufactured, distributed, or labeled by Target Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
UP AND UP NASAL- oxymetazoline hydrochloride spray
Target Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Target Corporation Nasal Spray Drug Facts
Uses
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily relieves sinus congestion and pressure
- shrinks swollen nasal membranes so you can breathe more freely
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: ask a doctor
To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use. Replace cap tightly to maintain child resistance.
| UP AND UP NASAL
oxymetazoline hydrochloride spray |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Target Corporation (006961700) |
Revised: 11/2019
Document Id: e29aa61f-6f5c-4150-b59f-88542c2abce4
Set id: 0c246c11-eadd-4ac6-bd58-5fdb151e06d6
Version: 5
Effective Time: 20191107
Target Corporation