GUAIFENESIN tablet, extended release
Guaifenesin by
Drug Labeling and Warnings
Guaifenesin by is a Otc medication manufactured, distributed, or labeled by WALGREEN CO., Aurohealth LLC, APL HEALTHCARE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1200 mg 14 (1 x 14) Tablets
Walgreens
Compare to Maximum Strength
Mucinex® active ingredient
NDC: 0363-0033-01
12 HOUR
Mucus Relief ER
GUAIFENESIN EXTENDED-RELEASE TABLETS
1200 MG / EXPECTORANT
MAXIMUM STRENGTH 12 HOUR- Relieves chest congestion
-
Thins and loosens mucus
14 69
EXTENDED-RELEASE ACTUAL SIZE
TABLETS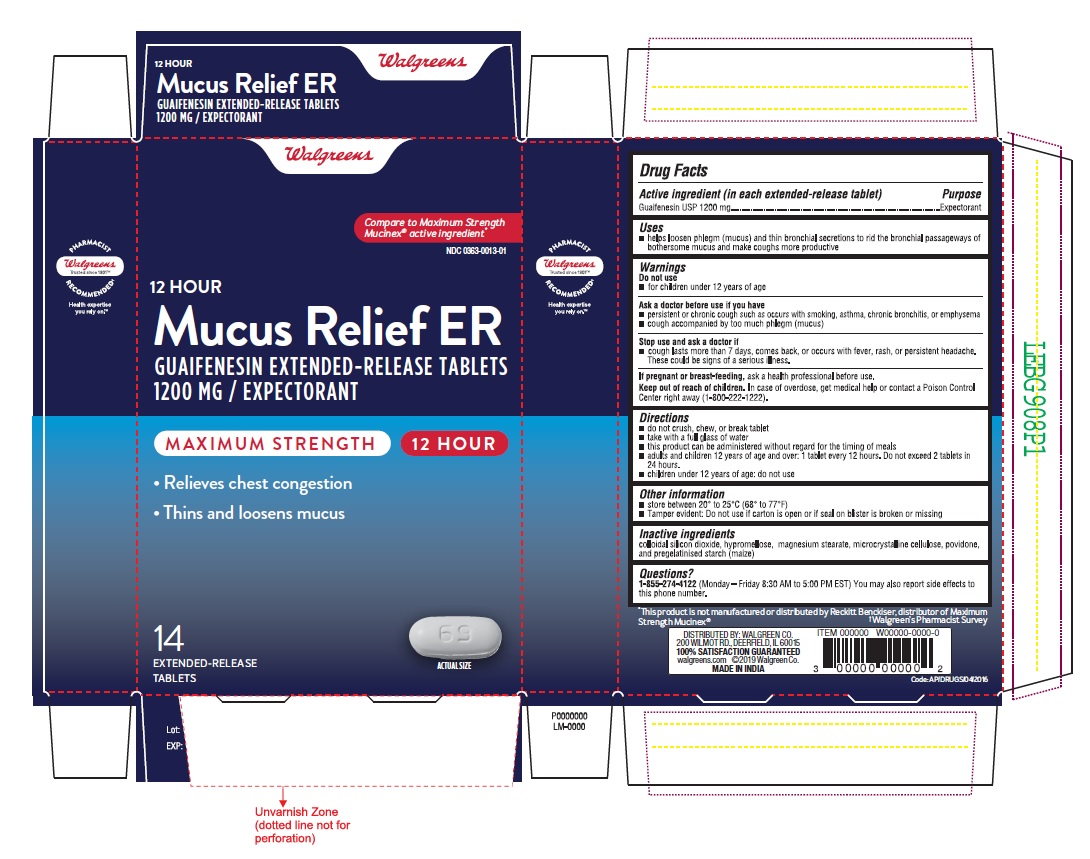
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0363-0033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POVIDONE K90 (UNII: RDH86HJV5Z) POVIDONE K25 (UNII: K0KQV10C35) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (white to off-white) Score no score Shape OVAL Size 21mm Flavor Imprint Code L;69 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0363-0033-01 1 in 1 CARTON 05/08/2020 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0363-0033-02 2 in 1 CARTON 05/08/2020 2 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210453 05/08/2020 Labeler - WALGREEN CO. (008965063) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650918514 ANALYSIS(0363-0033) , MANUFACTURE(0363-0033)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.