MACI- autologous cultured chondrocytes implant
MACI by
Drug Labeling and Warnings
MACI by is a Other medication manufactured, distributed, or labeled by Vericel Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MACI safely and effectively. See full prescribing information for MACI.
MACI® (autologous cultured chondrocytes on porcine collagen membrane)
Cellular sheet for autologous implantation
Initial U.S. Approval: 2016
INDICATIONS AND USAGE
MACI® is an autologous cellularized scaffold product indicated for the repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults. (1)
Limitations of Use
- Effectiveness of MACI in joints other than the knee has not been established.
- Safety and effectiveness of MACI in patients over the age of 55 years have not been established.
DOSAGE AND ADMINISTRATION
For autologous implantation only.
- Contact Vericel at 1-800-453-6948 or www.MACI.com regarding training materials for surgical implantation of MACI. (2)
- The amount of MACI implanted depends on the size (surface area in cm2) of the cartilage defect. (2.1)
- MACI should be trimmed to the size and shape of the defect and implanted with the cell-side down. (2.2)
DOSAGE FORMS AND STRENGTHS
Each 3 x 5 cm cellular sheet (MACI implant) consists of autologous cultured chondrocytes on a resorbable porcine Type I/III collagen membrane, at a density of at least 500,000 cells per cm2. (3)
CONTRAINDICATIONS
- Known history of hypersensitivity to gentamicin, other aminoglycosides, or products of porcine or bovine origin. (4)
- Severe osteoarthritis of the knee. (4)
- Inflammatory arthritis, inflammatory joint disease, or uncorrected congenital blood coagulation disorders. (4)
- Prior knee surgery (within 6 months), excluding surgery to procure a biopsy or a concomitant procedure to prepare the knee for a MACI implant. (4)
- Inability to cooperate with a physician-prescribed post-surgical rehabilitation program. (4)
WARNINGS AND PRECAUTIONS
- Safety of MACI in patients with malignancy in the area of cartilage biopsy or implant is unknown. Expansion of malignant or dysplastic cells present in biopsy tissue during manufacture and subsequent implantation may be possible. (5.1)
- Because patients undergoing procedures associated with MACI are not routinely tested for transmissible infectious diseases, cartilage biopsy and MACI implant may carry risk of transmitting infectious diseases. (5.2)
- Local inflammation or active infection in the bone, joint, and surrounding soft tissue, meniscal pathology, cruciate ligament instability, and misalignment should be assessed and treated prior to or concurrent with MACI implantation. (5.3)
- Final sterility test results are not available at the time of shipping. (5.4)
ADVERSE REACTIONS
The most frequently occurring adverse reactions (≥5%) reported for MACI were arthralgia, tendonitis, back pain, joint swelling, and joint effusion. (6)
Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Vericel at 1-800-453-6948 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch for voluntary reporting of adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy: Because MACI implantation requires invasive surgical procedures, use in pregnancy is not recommended. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Implantation Procedure
2.3 Postsurgical Rehabilitation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Malignancy
5.2 Transmissible Infectious Diseases
5.3 Presurgical Assessment of Comorbidities
5.4 Product Sterility
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
MACI® (autologous cultured chondrocytes on porcine collagen membrane) is an autologous cellularized scaffold product indicated for the repair of single or multiple symptomatic, full-thickness cartilage defects of the knee with or without bone involvement in adults.
Limitations of Use
- Effectiveness of MACI in joints other than the knee has not been established.
- Safety and effectiveness of MACI in patients over the age of 55 years have not been established.
-
2 DOSAGE AND ADMINISTRATION
For Autologous Implantation Only.
Contact Vericel at 1-800-453-6948 or www.MACI.com regarding training materials for surgical implantation of MACI.
2.1 Dosage
- The amount of MACI implanted depends on the size (surface area in cm2) of the cartilage defect. The surgeon should trim the MACI implant to the size and shape of the defect, to ensure the damaged area is completely covered.
- MACI implant is for single-use. Multiple implants may be used if there is more than one defect. The size of MACI is adjusted for the size of each cartilage defect.
2.2 Preparation and Implantation Procedure
Pre-Operative Preparation
- Confirm that the patient’s identity matches the patient identifiers on the MACI labels.
- Inspect the sealed MACI shipping box for any evidence of damage.
- Open the MACI shipping box and inspect the internal packaging for leaks (liquid) in the outer bag or self-seal pouch containing the covered dish holding the MACI implant or for any evidence of damage or contamination.
Note: Keep the dish upright at all times. - DO NOT USE if the patient identifiers do not match, or there are signs of leaking or damage to the packaging. Contact MACI representative immediately or call Vericel Customer Care at 1-800-453-6948.
- After inspection, keep MACI in its original packaging and store at room temperature until the surgical site has been prepared.
Implantation Procedure
- Perform implantation procedure during arthrotomy using sterile surgical techniques.
- Follow the implantation with an appropriate, physician-prescribed rehabilitation program [see Dosage and Administration (2.3)].
Preparing Defect
- For chondral defects, remove all damaged and fibrous tissue on the defect bed. Debride the defect bed back to stable cartilage with vertical walls down to the subchondral bone by removing as little healthy cartilage as possible (Figure 1). Do not penetrate the subchondral bone.
Figure 1: Preparing Defect Bed
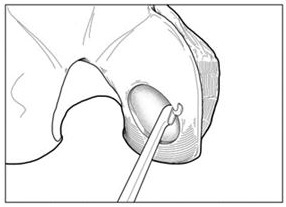
- For osteochondral defects, debride the defect bed back to stable cartilage with vertical walls down to healthy stable bone.
- Avoid bleeding through the subchondral plate. If bleeding occurs, use a suitable hemostatic agent to control the bleeding.
Creating Defect Template
- Create an exact template of the defect (Figure 2).
Figure 2: Creating Defect Template
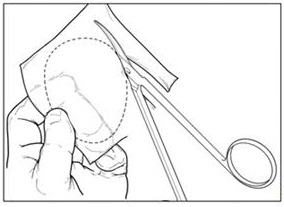
- Create orientation markers on the template to assist with proper orientation of the MACI implant. Turn the marked template over to ensure that the cells will be properly placed into the defect.
Preparing MACI Implant
- Unpacking MACI implant box (outside sterile field).
- – Unpack MACI implant shipping box.
- –
Remove the outer bag containing a covered dish holding the MACI implant.
Note: Keep the dish upright at all times. - –
Remove the self-seal pouch containing the dish from the outer bag (Figure 3).
Figure 3: Covered Dish in Self-Sealed Pouch
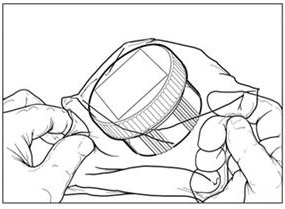
- –
Tear notches on the self-seal pouch to open the pouch and remove the covered dish.
Note: Do not remove the MACI implant from the dish until ready to be used.
- Unpacking the MACI implant dish (Figure 4)
- – When ready, a team member outside the sterile field but adjacent to the sterile prep table, will twist open and remove the lid from the dish.
- – Sterile field team member using sterile forceps will remove and discard the inner 5-pronged ring.
- –
Sterile field team member will use 2 sterile non-tooth forceps to grasp the MACI implant corners and place the MACI implant onto the sterile work surface.
Figure 4: Unpacking MACI Implant
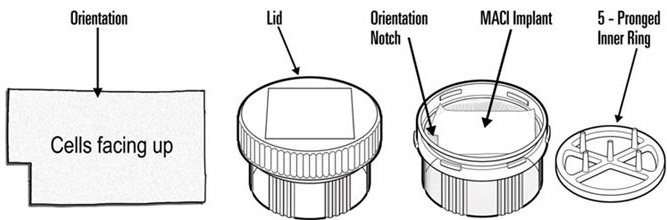
- –
The MACI implant has a rough side and a smooth side. The cells are seeded on the rough side and are facing up in the MACI dish. A notch in the lower left corner of the implant indicates that the cell-side is facing up. The cell-side of the MACI implant should remain facing up at all times until placement into the defect.
Note: The MACI implant must remain hydrated with the shipping media. Use the media from the dish to hydrate the implant if it ever starts to become dry after removal from the dish.
- Shaping the MACI implant
- –
To maintain proper orientation, turn the template over and place it underneath the MACI implant, against the smooth, non-seeded side. The template should be visible through the translucent implant.
Note: Ensure minimal contact with the cell-seeded surface of the MACI implant. - – Using the template as a guide, cut the MACI implant to the correct size and shape.
- – Place the custom-cut implant into a sterile intermediary dish, ensuring the cell-side up orientation and with adequate media from shipping dish to keep the implant hydrated.
- – Place any remaining MACI implant into a separate intermediary dish with adequate media from the shipping dish to keep the implant hydrated. Discard if unused by the end of the implantation.
- –
To maintain proper orientation, turn the template over and place it underneath the MACI implant, against the smooth, non-seeded side. The template should be visible through the translucent implant.
Placing MACI Implant
- Ensure defect area is dry and free of bleeding.
- Apply a thin layer of fibrin sealant to the entire base of the defect (bone) bed.
- Maintaining appropriate rotational orientation, place the custom-cut implant onto the defect bed cell-side down.
- Apply light digital pressure to the implant for approximately 3 minutes.
- Fibrin sealant may also be applied to the rim (periphery) of the implant. MACI implant fixation may also be supplemented with interrupted resorbable sutures if desired or if conditions warrant, particularly if the defect is uncontained (ie, the cartilage defect is not 100% surrounded by a stable cartilage rim) or the lesion is larger than 10 cm2.
2.3 Postsurgical Rehabilitation
A physician-prescribed rehabilitation program that includes early mobilization, joint range of motion, and weight bearing is recommended to promote graft maturation and reduce the risk of graft delamination, postoperative thromboembolic events, and joint stiffness. Stage this program to promote a progressive return to full joint range of motion and weight-bearing as well as muscle strengthening and conditioning. Return to recreational and sporting activity should be in consultation with healthcare professionals.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
MACI is contraindicated in patients with the following conditions:
- Known history of hypersensitivity to gentamicin, other aminoglycosides, or products of porcine or bovine origin. [see Description (11)]
- Severe osteoarthritis of the knee (Kellgren-Lawrence grade 3 or 4).
- Inflammatory arthritis, inflammatory joint disease, or uncorrected congenital blood coagulation disorders.
- Prior knee surgery (6 months), excluding surgery to procure a biopsy or a concomitant procedure to prepare the knee for a MACI implant.
- Inability to cooperate with a physician-prescribed post-surgical rehabilitation program [See Dosage and Administration (2.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Malignancy
The safety of MACI used in patients with malignancy in the area of cartilage biopsy or implant is unknown. The potential exists for expansion of malignant or dysplastic cells present in biopsy tissue during manufacture and subsequent implantation. In addition, implantation of normal autologous chondrocytes could theoretically stimulate growth of malignant cells in the area of the implant, although there have been no such incidents reported in humans or animals.
5.2 Transmissible Infectious Diseases
MACI is intended solely for autologous use. Patients undergoing the surgical procedures associated with MACI are not routinely tested for transmissible infectious diseases. Therefore, the cartilage biopsy and the MACI implant may carry the risk of transmitting infectious diseases to personnel handling these tissues. Accordingly, healthcare providers should employ universal precautions in handling the biopsy samples and the MACI product.
Product manufacture includes reagents derived from animal materials. All animal-derived reagents are tested for viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma before use. Bovine materials are sourced to minimize the risk of transmitting a prion protein that causes bovine spongiform encephalopathy and may cause a rare fatal condition in humans called variant Creutzfeldt-Jakob disease.
These measures do not totally eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents. Report the occurrence of a transmitted infection to Vericel Corporation at 1-800-453-6948.
5.3 Presurgical Assessment of Comorbidities
To create a favorable environment for healing, assess and treat the following conditions prior to or concurrent with implantation with MACI:
- Local inflammation or active infection in the bone, joint, and surrounding soft tissue: patients should be deferred until complete recovery.
- Meniscal pathology: presence of an unstable or torn meniscus requires partial resection, repair, or replacement prior to or concurrent with MACI implantation. MACI is not recommended in patients with a total meniscectomy.
- Cruciate ligament instability: the joint should not possess excessive laxity, which may create excessive shear and rotational forces across the joint. Both anterior and posterior cruciate ligaments should be stable or undergo reconstruction prior to or concurrent with MACI implantation.
- Misalignment: the tibio-femoral joint should be properly aligned, and patella tracking should be normalized. Varus or valgus misalignment of the tibio-femoral joint and abnormal patella tracking may abnormally load joint surfaces and jeopardize the implant. Misalignment and patella tracking should be addressed with a corrective osteotomy or similar corrective procedure prior to or concurrent with MACI implantation.
5.4 Product Sterility
MACI is shipped after passing preliminary test results from in-process microbial tests. A final sterility test is initiated prior to shipping, but the result will not be available prior to implantation. If microbial contamination is detected after the product has been shipped, Vericel will notify the healthcare provider(s) and recommend appropriate actions.
-
6 ADVERSE REACTIONS
The most frequently occurring adverse reactions (≥5%) reported for MACI were arthralgia, tendonitis, back pain, joint swelling, and joint effusion.
Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
In a 2-year prospective, multicenter, randomized, open-label, parallel-group clinical trial1, 144 patients, ages 18 to 54 years, were randomized to receive a 1-time treatment with MACI or microfracture (1:1, 72 patients in each treatment group). Demographic characteristics of patients in the trial were similar in both treatment groups. The majority of patients were male (62.5% MACI, 66.7% microfracture), and the mean ages were 34.8 (MACI) and 32.9 (microfracture) years. Overall, 70 patients in the MACI group and 67 patients in the microfracture group completed 2 years of follow-up.
In addition, all 144 subjects from the 2-year clinical trial had the option to enroll in a 3-year follow-up study (extension study). Safety and efficacy assessments were performed at yearly scheduled visits. The demographic characteristics of patients (N = 128) enrolled in the extension study were similar in both treatment groups and consistent with the overall population of the 2-year clinical trial.
The proportion of patients with at least 1 subsequent surgical procedure (any surgical procedure performed on the treated knee joint, including arthroscopy, arthrotomy, or manipulation under anesthesia) in the 2 years following study treatment was comparable between treatment groups (8.3% in the MACI group and 9.7% in the microfracture group).
Adverse reactions reported in ≥5% of patients in either treatment group in the 2-year clinical trial are provided in Table 1.
Table 1. Adverse Reactions in ≥5% of Patients in Any Treatment Group in the 2-Year Clinical Trial System Organ Class MACI
n = 72
n (%)Microfracture
n = 72
n (%)Musculoskeletal and Connective Tissue Disorders Arthralgia 37 (51.4) 46 (63.9) Back pain 8 (11.1) 7 (9.7) Joint swelling 7 (9.7) 4 (5.6) Joint effusion 5 (6.9) 4 (5.6) Injury, Poisoning and Procedural Complications Cartilage injury 3 (4.2) 9 (12.5) Ligament sprain 3 (4.2) 5 (6.9) Procedural pain 3 (4.2) 4 (5.6) General Disorders and Administration Site Conditions Treatment failure 1 (1.4) 4 (5.6) In the 3-year extension study, adverse reactions reported in ≥5% of patients were (MACI vs microfracture): arthralgia (46.2% vs 50.8%), tendonitis (6.2% vs 1.6%), back pain (4.6% vs 6.3%), osteoarthritis (4.6% vs 7.9%), joint effusion (3.1% vs 7.9%), cartilage injury (6.2% vs 15.9%), procedural pain (3.1% vs 7.9%), ligament sprain (1.5% vs 7.9%), and treatment failure (4.6% vs 7.9%).
Serious adverse reactions reported in patients in either treatment group for integrated data across the 2-year clinical trial and the 3-year extension study are provided in Table 2.
Table 2. Serious Adverse Reactions in Patients in Any Treatment Group Across the 2-Year Clinical Trial and the 3-Year Extension Study System Organ Class MACI
n = 72
n (%)Microfracture
n = 72
n (%)Musculoskeletal and Connective Tissue Disorders Arthralgia 1 (1.4) 7 (9.7) Joint Lock 0 3 (4.2) Meniscus Injury 3 (4.2) 0 Osteoarthritis 3 (4.2) 0 Injury, Poisoning and Procedural Complications Cartilage injury 3 (4.2) 8 (11.1) General Disorders and Administration Site Conditions Treatment failure 3 (4.2) 7 (9.7) 6.2 Postmarketing Experience
Graft complication (e.g., abnormalities to the repair graft that become symptomatic; this could include graft overgrowth [tissue hypertrophy], under-fill or damage to the repair tissue that has elicited a painful response, or mechanical symptoms), graft delamination (i.e., a dislodging of the repair graft from the underlying subchondral bone that has become symptomatic; this can be measured as marginal, partial, or a complete delaminated graft), and tendonitis have been reported during use of MACI outside the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to MACI exposure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
MACI implantation requires invasive surgical procedures; therefore use during pregnancy is not recommended. Limited clinical data on patients exposed to MACI during pregnancy are available. There are insufficient data with MACI use in pregnant women to inform a product-associated risk. Animal reproduction studies have not been conducted with MACI. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of MACI in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MACI and any potential adverse effects on the breastfed infant from MACI or from the underlying maternal condition.
-
11 DESCRIPTION
MACI, autologous cultured chondrocytes on porcine collagen membrane, is a cellular sheet that consists of autologous chondrocytes seeded on a 3 x 5 cm, resorbable porcine Type I/III collagen membrane, for implantation into cartilage defects of the knee. The active ingredients of MACI are the autologous cultured chondrocytes and porcine Type I/III collagen. The autologous chondrocytes are propagated in cell culture and are seeded on the collagen at a density of 500,000 to 1,000,000 cells per cm2. The final MACI implant contains at least 500,000 cells per cm2 and does not contain any preservative.
The product manufacture also uses reagents derived from animal materials. The resorbable, Type I/III, collagen membrane, which is a component of MACI, is porcine-derived. Fetal bovine serum is a component in the culture medium used to propagate the autologous chondrocytes; therefore, trace quantities of bovine-derived proteins may be present in MACI. These animal-derived reagents are tested for viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma before use.
MACI may contain residual gentamicin because it is included during manufacture. Gentamicin is not included in the transport medium used to maintain product stability. Studies determined an average of 9.2 μg residual gentamicin per MACI implant.
A final sterility test is initiated prior to shipping, but the result will not be available prior to implantation. Passing results from preliminary in-process microbial tests are required for release of MACI for shipping.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to evaluate the carcinogenicity or impairment of fertility potential of MACI have not been performed. In vitro studies have shown that the expansion process for chondrocytes does not induce changes to the cellular karyotype.
Four studies (in vitro and in vivo) were conducted to assess the genotoxic potential of the collagen membrane. The results from these studies demonstrated that the collagen membrane was non-mutagenic.
13.2 Animal Toxicology and/or Pharmacology
Implantation of analogous products in critical-size defects in the hind limbs of rabbits and horses did not reveal any serious safety concerns. The products consisted of the same membrane as MACI with rabbit or horse cells, respectively. Non-clinical testing has shown that the collagen membrane is not toxic and is compatible with biological tissue.
-
14 CLINICAL STUDIES
The effectiveness of MACI implant was evaluated in a 2-year prospective, multicenter, randomized, open-label, parallel-group study, SUMMIT (Superiority of MACI implant versus Microfracture Treatment in patients with symptomatic articular cartilage defects in the knee),1 which enrolled a total of 144 subjects, ages 18 to 54 years, with at least one symptomatic Outerbridge Grade III or IV focal cartilage defect on the medial femoral condyle, lateral femoral condyle, and/or the trochlea. Failure of a prior cartilage surgery was not required for study entry. The subjects were randomized to receive either a 1-time treatment with MACI or microfracture. The co-primary efficacy endpoint was change from baseline to Week 104 for the subject’s Knee injury and Osteoarthritis Outcome Score (KOOS) in two subscales: Pain and Function (Sports and Recreational Activities [SRA])2. Safety also was evaluated through Week 104 [see Adverse Reactions (6.1)].
Of the 72 subjects randomized to MACI, 70 completed the study and 2 discontinued prematurely (1 due to an adverse event [AE] and 1 wished to withdraw). Of the 72 subjects randomized to microfracture, 67 completed the study and 5 discontinued prematurely (1 due to an AE, 1 wished to withdraw, and 3 due to lack of clinical benefit).
At Week 104, KOOS pain and function (SRA) had improved from baseline in both treatment groups, but the improvement was statistically significantly (p = 0.001) greater in the MACI group compared with the microfracture group (Table 3).
Table 3. Change in KOOS Pain and Function (SRA) Scores in the 2-Year Study LS = least squares; KOOS = Knee injury and Osteoarthritis Outcome Score; SD = standard deviation; SRA = Sports and Recreational Activities.
* Difference in least squares mean values at Week 104 [MACI – Microfracture].
**p-value for difference in co-primary endpoints assessed jointly at Week 104 based on multivariate analysis of variance.MACI
Mean (SD)Microfracture
Mean (SD)N Pain Function N Pain Function Baseline 72 37.0 (13.5) 14.9 (14.7) 71 35.4 (12.1) 12.6 (16.7) Week 104 72 82.4 (16.2) 60.9 (27.8) 70 70.9 (24.2) 48.7 (30.3) Change From Baseline to Week 104 72 45.4 (21.1) 46.0 (28.4) 69 35.2 (23.9) 35.8 (31.6) LS Means (Week 104) 44.1 46.1 32.4 34.6 Difference * [MACI – Microfracture] 11.8 11.4 p-value ** 0.001 In a responder analysis, the proportion of subjects with at least a 10-point improvement in both KOOS pain and function (SRA) was greater in the MACI group (63/72=87.5%; 95% CI [77.6%, 94.1%]) compared with the microfracture group (49/72=68.1%; 95% CI [56.0%, 78.6%]).
All subjects from the 2-year study had the option to enroll in a 3 year follow-up study (extension study), in which 128 subjects participated. All 65 subjects (100%, 65/65) in the MACI group and 59 subjects (93.7%, 59/63) in the microfracture group completed the extension study. The mean 2-year KOOS pain and function scores remained stable for the additional 3-year period in both treatment groups (Table 4).
Table 4. KOOS Pain and Function (SRA) Scores in the 3-Year Extension Study Visit MACI Microfracture N Pain
mean (SD)Function
mean (SD)N Pain
mean (SD)Function
mean (SD)Baseline 65/65 37.1 (13.1) 15.4 (14.8) 63/63 35.2 (12.3) 11.9 (16.2) 2 Years 63/63 82.2 (15.8) 60.5 (26.5) 60/60 71.8 (23.9) 48.9 (30.6) 5 Years 65/64 82.2 (20.1) 61.9 (30.9) 59/59 74.8 (21.7) 50.3 (32.3) -
15 REFERENCES
- Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014 Jun;42(6):1384-94.
- Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- A single patient order may contain 1 or 2 implants, each in its own dish and shipper, depending on lesion size and number of lesions.
- MACI, NDC69866-1030-1, contains 1 implant supplied ready for use as a single cellular sheet approximately 3 x 5 cm, in a sterile, sealed, clear polystyrene dish. Each dish contains one 3 x 5 cm implant with a 0.5 cm2 section removed from the lower left-hand corner, held in place by a polycarbonate 5-pronged ring closed with a polycarbonate cover for shipment.
- MACI, NDC69866-1030-2, contains 2 implants supplied ready for use as cellular sheets approximately 3 x 5 cm, in a sterile, sealed, clear polystyrene dish. Each dish contains one 3 x 5 cm implant with a 0.5 cm2 section removed from the lower left-hand corner, held in place by a polycarbonate 5-pronged ring closed with a polycarbonate cover for shipment.
- Each dish is individually sealed in a clear self-seal pouch. The self-seal pouch is placed into one 95kPa outer bag with absorbent material. These bags are enclosed in an outer carton insulated with ambient temperature gel packs.
- MACI is shipped cell-side up.
Storage and Handling
- Store MACI at room temperature in its original packaging (outer carton) until ready to use.
- DO NOT REFRIGERATE or FREEZE, or sterilize MACI.
- DO NOT USE if the dish is damaged, has been compromised, or has leaked.
- Use MACI prior to 11:59 PM ET on the date of expiration printed on the package.
- Dispose of unused MACI or waste material as surgical biohazardous waste in accordance with local requirements.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient that:
- – A cartilage biopsy is needed to manufacture MACI. The biopsy is typically performed as an arthroscopic procedure at the time of diagnosis confirmation.
- – The length of time between the biopsy and the implantation of MACI may vary depending on many factors, including the quality and number of cells obtained from the biopsy. On average this will take 6 weeks; however, cells can be held in storage until a convenient date for surgery is agreed upon between the patient and the surgeon.
- – Even if the surgeon has taken a biopsy needed to produce MACI, it may be possible that the patient cannot be treated with MACI, (e.g., in case the biopsy is of insufficient quality to produce MACI, if the cells cannot be grown in the laboratory, or if the expanded cells do not meet all the quality requirements).
- Advise the patient on the risk of graft complications, subsequent surgical procedures, and treatment failure. [See Adverse Reactions (6)]
- Advise the patient on general complications related to knee surgery, which may include deep vein thrombosis and pulmonary embolism.
- Advise the patient to closely follow the physician-prescribed rehabilitation program, which will include limitations and allowances for beginning specific physical activities. [See Dosage and Administration (2.3)]
Manufactured by: Vericel Corporation, 64 Sidney Street, Cambridge, MA 02139
MACI® is a registered trademark of Vericel Corporation.
Patents: www.vcel.com/research-and-development
© 2018 Vericel Corporation.
65617.D
- Advise the patient that:
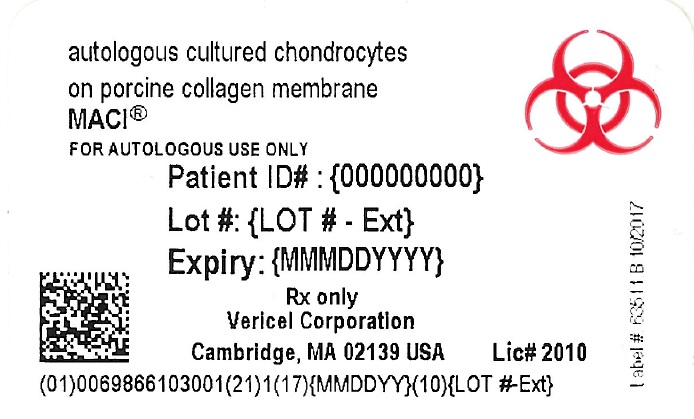
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-1 - Dish Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-1 - Outer Bag Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-1 - Shipper Label
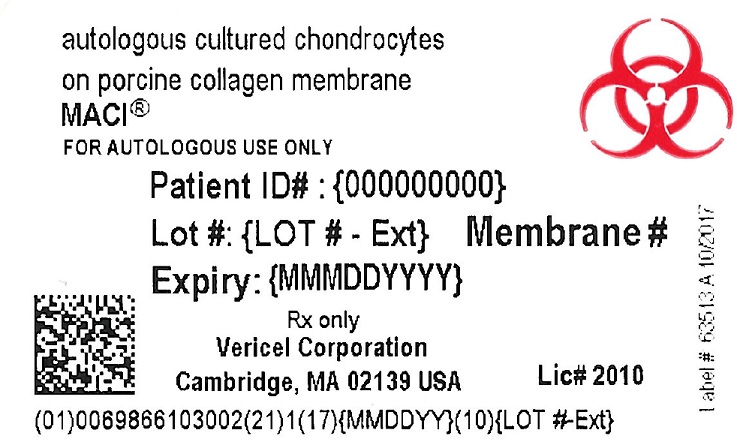
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-2 - Dish Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-2 - Outer Bag Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-1030-2 - Shipper Label
-
INGREDIENTS AND APPEARANCE
MACI
autologous cultured chondrocytes implantProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC: 69866-1030 Route of Administration INTRA-ARTICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AUTOLOGOUS CULTURED CHONDROCYTES (UNII: D5P3K3V822) (AUTOLOGOUS CULTURED CHONDROCYTES - UNII:D5P3K3V822) AUTOLOGOUS CULTURED CHONDROCYTES 15000000 PORK COLLAGEN (UNII: I8442U2G7J) (PORK COLLAGEN - UNII:I8442U2G7J) PORK COLLAGEN 15 cm2 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-1030-1 1 in 1 BOX 1 1 in 1 POUCH 1 1 in 1 CONTAINER; Type 5: Device Coated or Otherwise Combined with Biologic 2 NDC: 69866-1030-2 1 in 1 BOX 2 2 in 1 POUCH 2 1 in 1 CONTAINER; Type 5: Device Coated or Otherwise Combined with Biologic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125603 01/11/2017 Labeler - Vericel Corporation (079745570)
Trademark Results [MACI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MACI 87035415 5108912 Live/Registered |
Southern States LLC 2016-05-12 |
 MACI 86855486 5770006 Live/Registered |
VERICEL CORPORATION 2015-12-21 |
 MACI 86640793 not registered Dead/Abandoned |
Scott M. Schlesinger, MD, FACS 2015-05-26 |
 MACI 85843354 not registered Dead/Abandoned |
VERICEL CORPORATION 2013-02-07 |
 MACI 85611711 4261148 Live/Registered |
Dicandrien, Inc. 2012-04-30 |
 MACI 85512439 4927713 Live/Registered |
VERICEL CORPORATION 2012-01-10 |
 MACI 77739920 not registered Dead/Abandoned |
GENZYME CORPORATION 2009-05-19 |
 MACI 75854103 2535450 Dead/Cancelled |
Verigen Transplantation Service International (VTSI) AG 1999-11-19 |
 MACI 74041633 1631438 Dead/Cancelled |
Regulatory Resources Inc. 1990-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.