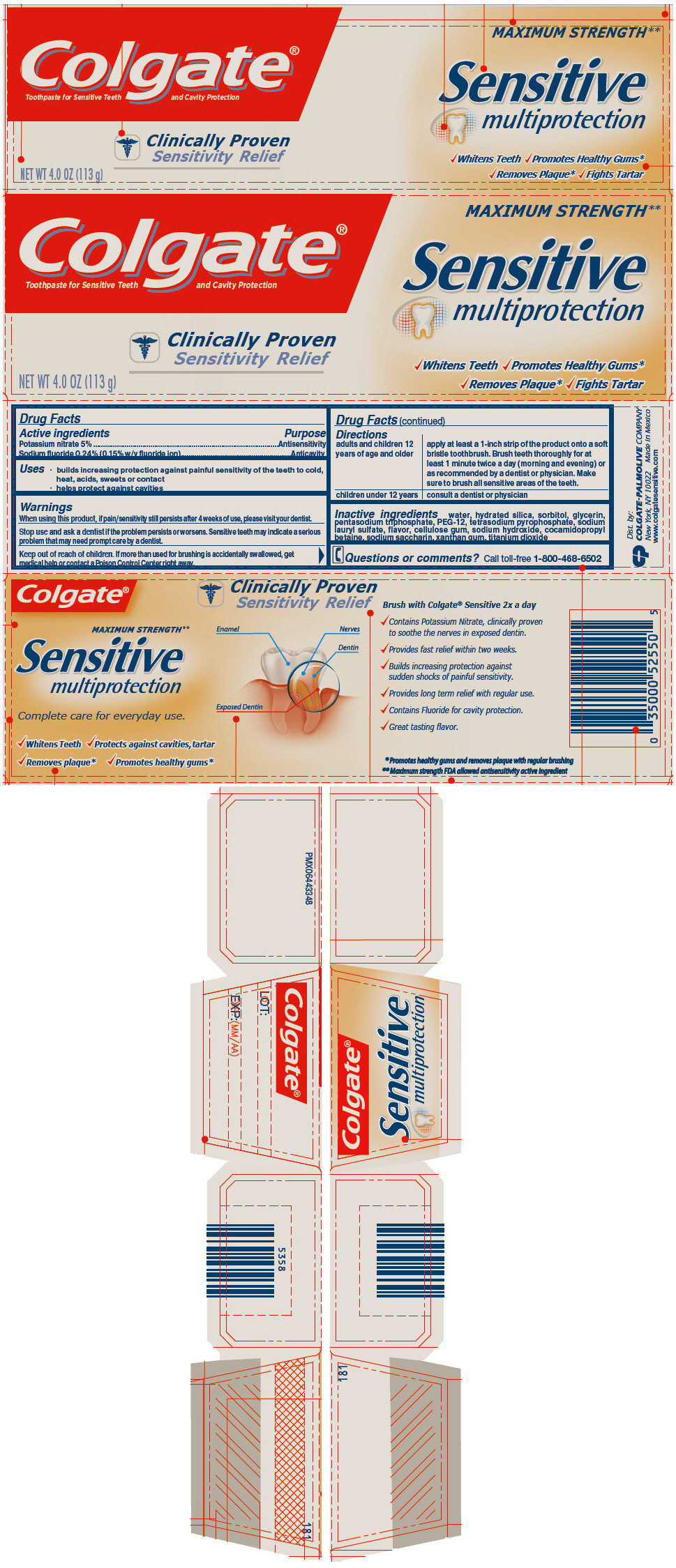
Colgate® Sensitive Multiprotection Fluoride Toothpaste 4.0 oz
Colgate Sensitive Multiprotection by
Drug Labeling and Warnings
Colgate Sensitive Multiprotection by is a Otc medication manufactured, distributed, or labeled by Mission Hills S.A de C.V. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COLGATE SENSITIVE MULTIPROTECTION- sodium fluoride and potassium nitrate paste, dentifrice
Mission Hills S.A de C.V
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Colgate® Sensitive Multiprotection Fluoride Toothpaste 4.0 oz
Uses
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
- helps protect against cavities
Warnings
When using this product, if pain/sensitivity still persists after 4 weeks of use, please visit your dentist.
Directions
| adults and children 12 years of age and older | apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth. |
| children under 12 years | consult a dentist or physician |
| COLGATE SENSITIVE MULTIPROTECTION
sodium fluoride and potassium nitrate paste, dentifrice |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Mission Hills S.A de C.V (812312122) |
Revised: 4/2019
Document Id: 4cdf7b1c-a409-4a32-a277-310893e79f0e
Set id: 0c3b1afd-6d99-443c-8d27-87feffea1c82
Version: 2
Effective Time: 20190429