AZASITE- azithromycin monohydrate solution/ drops
AZASITE by
Drug Labeling and Warnings
AZASITE by is a Prescription medication manufactured, distributed, or labeled by Thea Pharma Inc., Woodstock Sterile Solutions, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AzaSite safely and effectively. See full prescribing information for AzaSite.
AzaSite ® (azithromycin ophthalmic solution) 1%
Sterile topical ophthalmic drops
Initial U.S. Approval: 2007INDICATIONS AND USAGE
AzaSite is a macrolide antimicrobial indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following microorganisms: CDC coryneform group G, Haemophilus influenzae, Staphylococcus aureus, Streptococcus mitis group, and Streptococcus pneumoniae. ( 1)
DOSAGE AND ADMINISTRATION
Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye(s) once daily for the next five days. ( 2)
DOSAGE FORMS AND STRENGTHS
2.5 mL of 1% sterile topical ophthalmic solution. ( 3)
CONTRAINDICATIONS
Hypersensitivity ( 4)
WARNINGS AND PRECAUTIONS
- For topical ophthalmic use only. ( 5.1)
- Anaphylaxis and hypersensitivity have been reported with systemic use of azithromycin. ( 5.2)
- Growth of resistant organisms may occur with prolonged use. ( 5.3)
- Patients should not wear contact lenses if they have signs or symptoms of bacterial conjunctivitis. ( 5.4)
ADVERSE REACTIONS
Most common adverse reaction reported in patients was eye irritation (1 to 2% of patients). ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Thea Pharma Inc. at 1-833-838-4028 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
5.2 Anaphylaxis and Hypersensitivity with Systemic Use of Azithromycin
5.3 Growth of Resistant Organisms with Prolonged Use
5.4 Contamination of the Applicator Tip
5.5 Avoidance of Contact Lenses
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
AzaSite ® is indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following microorganisms:
CDC coryneform group G 1
Haemophilus influenzae
Staphylococcus aureus
Streptococcus mitis group
Streptococcus pneumoniae
- 1 Efficacy for this organism was studied in fewer than 10 infections.
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage regimen for the treatment of bacterial conjunctivitis is:
Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye(s) once daily for the next five days.
2.1 Recommended Dosage
The recommended dosage for the treatment of bacterial conjunctivitis is:
Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye(s) once daily for the next five days.
2.2 Administration Instructions
Wash hands prior to using AzaSite.
Invert the closed bottle (upside down) and shake once before each use. Remove the tan cap with the bottle still in the inverted position. Tilt head back, and with bottle inverted, gently squeeze bottle to instill one drop into the affected eye(s).
Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by AzaSite (azithromycin ophthalmic solution) or other antibacterial drugs in the future.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
NOT FOR INJECTION. AzaSite is indicated for topical ophthalmic use only, and should not be administered systemically, injected subconjunctivally, or introduced directly into the anterior chamber of the eye.
5.2 Anaphylaxis and Hypersensitivity with Systemic Use of Azithromycin
In patients receiving systemically administered azithromycin, serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported rarely in patients on azithromycin therapy. Although rare, fatalities have been reported. The potential for anaphylaxis or other hypersensitivity reactions should be considered based on known hypersensitivity to azithromycin when administered systemically.
5.3 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If super-infection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and where appropriate, fluorescein staining.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with the rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to AzaSite in 698 patients. The population was between 1 and 87 years old with clinical signs and symptoms of bacterial conjunctivitis. The most frequently reported ocular adverse reaction reported in patients receiving AzaSite was eye irritation. This reaction occurred in approximately 1 to 2% of patients. Other adverse reactions associated with the use of AzaSite were reported in less than 1% of patients and included ocular reactions (blurred vision, burning, stinging and irritation upon instillation, contact dermatitis, corneal erosion, dry eye, eye pain, itching, ocular discharge, punctate keratitis, visual acuity reduction) and non-ocular reactions (dysgeusia, facial swelling, hives, nasal congestion, periocular swelling, rash, sinusitis, urticaria).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes ( seeData). Developmental toxicity studies with azithromycin in rats, mice, and rabbits showed no drug-induced fetal malformations at doses up to 200 mg/kg/day. The doses used in these studies were orders of magnitude in excess of the clinical exposure that would be possible following topical ocular administration of azithromycin. ( see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Available data from published observational studies, case series, and case reports over several decades do not suggest an increased risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes with oral or topical ophthalmic azithromycin use in pregnant women. Limitations of these data include the lack of randomization and inability to control for confounders such as underlying maternal disease and maternal use of concomitant medications.
Animal Data
Azithromycin administered during the period of organogenesis did not cause fetal malformations in rats and mice at oral doses up to 200 mg/kg/day (moderately maternally toxic). Assuming 100% absorption from topical ocular exposure, this dose is hundreds of times daily dose that can be achieved with topical ophthalmic administration. In rabbits administered azithromycin at oral doses of 10, 20, and 40 mg/kg/day during organogenesis, reduced maternal body weight and food consumption were observed in all groups; no evidence of fetotoxicity or teratogenicity was observed at these doses, the highest of which is estimated to be at least 100 times topical ophthalmic dose assuming 100% absorption from ocular exposure.
In a pre- and postnatal development study, azithromycin was administered orally to pregnant rats from day 6 of pregnancy until weaning at doses of 50 or 200 mg/kg/day. Maternal toxicity (reduced food consumption and body weight gain; increased stress at parturition) was observed at the higher dose. Effects in the offspring were noted at 200 mg/kg/day during the postnatal development period (decreased viability, delayed developmental landmarks). These effects were not observed in a pre- and postnatal rat study when up to 200 mg/kg/day of azithromycin was given orally beginning on day 15 of pregnancy until weaning. Assuming 100% absorption from topical ocular exposure, this dose is hundreds of times daily dose that can be achieved with topical ophthalmic administration.
8.2 Lactation
Risk Summary
Azithromycin is present in human milk ( see Data). Non-serious adverse reactions have been reported in breastfed infants after maternal administration of oral azithromycin. There are no available data on the effects of azithromycin on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AzaSite and any potential adverse effects on the breastfed infant from AzaSite.
Data
Azithromycin breastmilk concentrations were measured in 20 women after receiving a single 2 g oral dose of azithromycin during labor. Breastmilk samples collected on days 3 and 6 postpartum as well as 2 and 4 weeks postpartum revealed the presence of azithromycin in breastmilk up to 4 weeks after dosing. In another study, a single dose of azithromycin 500 mg was administered intravenously to 8 women prior to incision for cesarean section. Breastmilk (colostrum) samples obtained between 12 and 48 hours after dosing revealed that azithromycin persisted in breastmilk up to 48 hours.
8.4 Pediatric Use
The safety and effectiveness of AzaSite solution in pediatric patients have been established. The efficacy of AzaSite in treating bacterial conjunctivitis in pediatric patients has been demonstrated in controlled clinical trials [see Clinical Studies (14)] .
-
11 DESCRIPTION
AzaSite® (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite ® (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of approximately 290 mOsm/kg.
Preservative: 0.003% benzalkonium chloride. Inactives: mannitol, citric acid, sodium citrate, poloxamer 407, polycarbophil, edetate disodium (EDTA), sodium chloride, water for injection, and sodium hydroxide to adjust pH to 6.3.
Azithromycin is a macrolide antibiotic with a 15-membered ring. Its chemical name is (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-aza-cyclopentadecan-15-one, and the structural formula is:
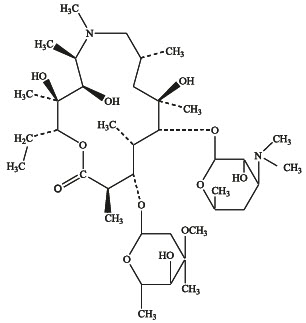
Azithromycin has a molecular weight of 749, and its empirical formula is C 38H 72N 2O 12.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Azithromycin is a macrolide antibiotic [see Clinical Pharmacology (12.4)] .
12.3 Pharmacokinetics
The plasma concentration of azithromycin following ocular administration of AzaSite (azithromycin ophthalmic solution) in humans is unknown. Based on the proposed dose of one drop to each eye (total dose of 100 mcL or 1 mg) and exposure information from systemic administration, the systemic concentration of azithromycin following ocular administration is estimated to be below quantifiable limits (≤10 ng/mL) at steady-state in humans, assuming 100% systemic availability.
12.4 Microbiology
Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and interfering with microbial protein synthesis.
Azithromycin has been shown to be active against most isolates of the following microorganisms, both in vitro and clinically in conjunctival infections [see Indications and Usage (1)] .
CDC coryneform group G 2
Haemophilus influenzae
Staphylococcus aureus
Streptococcus mitis group
Streptococcus pneumoniaeThe following in vitro data are also available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of AzaSite in treating ophthalmological infections due to these microorganisms have not been established.
The following microorganisms are considered susceptible when evaluated using systemic breakpoints. However, a correlation between the in vitro systemic breakpoint and ophthalmological efficacy has not been established. This list of microorganisms is provided as an aid only in assessing the potential treatment of conjunctival infections. Azithromycin exhibits in vitro minimal inhibitory concentrations (MICs) of equal or less (systemic susceptible breakpoint) against most (≥90%) of isolates of the following ocular pathogens:
Chlamydia pneumoniae
Chlamydia trachomatis
Legionella pneumophila
Moraxella catarrhalis
Mycoplasma hominis
Mycoplasma pneumoniae
Neisseria gonorrhoeae
Peptostreptococcus species
Streptococci (Groups C, F, G)
Streptococcus pyogenes
Streptococcus agalactiae
Ureaplasma urealyticum
Viridans group streptococci
- 2 Efficacy for this organism was studied in fewer than 10 infections.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. No evidence of impaired fertility due to azithromycin was found in mice or rats that received oral doses of up to 200 mg/kg/day.
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) has been observed in some tissues of mice, rats, and dogs given multiple systemic doses of azithromycin. Cytoplasmic microvacuolation, which is likely a manifestation of phospholipidosis, has been observed in the corneas of rabbits given multiple ocular doses of AzaSite. This effect was reversible upon cessation of AzaSite treatment. The significance of this toxicological finding for animals and for humans is unknown.
-
14 CLINICAL STUDIES
In a randomized, vehicle-controlled, double-blind, multicenter clinical study in which patients were dosed twice daily for the first two days, then once daily on days 3, 4, and 5, AzaSite solution was superior to vehicle on days 6 to 7 in patients who had a confirmed clinical diagnosis of bacterial conjunctivitis. Clinical resolution was achieved in 63% (82/130) of patients treated with AzaSite versus 50% (74/149) of patients treated with vehicle. The p-value for the comparison was 0.03 and the 95% confidence interval around the 13% (63% to 50%) difference was 2% to 25%. The microbiological success rate for the eradication of the baseline pathogens was approximately 88% compared to 66% of patients treated with vehicle (p<0.001, confidence interval around the 22% difference was 13% to 31%). Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
AzaSite® is a sterile aqueous topical ophthalmic formulation of 1% azithromycin.
NDC: 82584-307-03: 2.5 mL in 5 mL bottle containing a total of 25 mg of azithromycin in a white, oblong, low-density polyethylene (LDPE) bottle, with a clear LDPE dropper tip, and a tan colored high density polyethylene (HDPE) eyedropper cap. A white tamper evident over-cap is provided.
Storage and Handling
Store unopened bottle under refrigeration at 2° to 8°C (36° to 46°F). Once the bottle is opened, store at 2° to 25°C (36° to 77°F) for up to 14 days. Discard after the 14 days.
The white tamper evident over-cap can be thrown away. Retain the tan cap and keep the bottle tightly closed when not in use.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling ( Patient Information and Instructions for Use).
Avoiding Contamination of the Applicator Tip
Advise patients to avoid contaminating the applicator tip by not allowing it to touch the eye, fingers or other sources.
When to Seek Physician Advice
Direct patients to discontinue use and contact a physician if any signs of an allergic reaction occur.
Tell patients that although it is common to feel better early in the course of the therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by AzaSite (azithromycin ophthalmic solution) or other antibacterial drugs in the future.
Contact Lens Use
Advise patients not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis.
Administration Instructions
Advise patients to thoroughly wash hands prior to using AzaSite.
Advise patients to invert the closed bottle (upside down) and shake once before each use. Remove cap with bottle still in the inverted position. Tilt head back, and with bottle inverted, gently squeeze bottle to instill one drop into the affected eye(s).
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION
AzaSite ® (A-zuh-site)
(azithromycin ophthalmic solution) 1%Read this Patient Information before you start using AzaSite ® and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
What is AzaSite?
AzaSite is a prescription sterile eye drop solution. AzaSite is used to treat bacterial conjunctivitis which is an infection of the eye caused by certain bacteria.
Information about bacterial conjunctivitis.
Bacterial conjunctivitis is a bacterial infection of the mucous membranes which line the inside of the eyelids. Symptoms may include redness of the eye and discharge. The infection can be spread to other people and to both eyes.
Who should not use AzaSite?
Do not use AzaSite if you are allergic to azithromycin or any of the ingredients in AzaSite. See the end of this Patient Information leaflet for a complete list of the ingredients in AzaSite.
What should I tell my doctor before using AzaSite?
Before you use AzaSite, tell your doctor if you:
- wear contact lenses. Do not wear contact lenses if you have signs or symptoms of bacterial conjunctivitis.
- are pregnant or plan to become pregnant. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. AzaSite passes into your breast milk. Talk to your doctor about the best way to feed your baby if you are using AzaSite.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctors and pharmacist when you get a new medicine.
How should I use AzaSite?
- Read the Instructions for Use at the end of this Patient Information leaflet for the right way to use AzaSite.
- Use AzaSite exactly as your doctor tells you to use it.
- For the first 2 days place 1 drop of AzaSite in your eye (or eyes) each morning and 1 drop in your eye (or eyes) each evening. Wait 8 to 12 hours after placing your morning drops before you place evening drops in your eye (or eyes).
- For the next 5 days place 1 drop of AzaSite in your eye (or eyes) 1 time each day.
- Make sure you continue to use AzaSite as directed by your doctor even if you feel better after you start using it. Skipping drops can increase the chances that:
- your medicine will not work well
- Bacteria can develop resistance, which means in the future your bacterial conjunctivitis may not improve from AzaSite or other drugs that treat infections from bacteria.
What should I be aware of while using AzaSite?
Do not wear contact lenses if you have signs or symptoms of bacterial conjunctivitis and until you have finished your prescribed course of treatment. The symptoms of bacterial conjunctivitis may include:
- discharge coming from the eye
- eye redness
- eye irritation
Only your doctor can tell you if you have bacterial conjunctivitis.
Severe allergic reactions have been reported rarely when azithromycin has been taken by mouth.
- Serious rash or serious allergic reactions may occur. Azithromycin, the active ingredient in AzaSite, may cause a serious rash or a serious allergic reaction. Both of these reactions may need to be treated in a hospital and may be life-threatening.
- Stop taking AzaSite and call your doctor right away or get emergency help if you have any of these symptoms:
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- swelling of your face, eyes, lips, tongue, or throat
- trouble swallowing or breathing
Increased risk of other infections caused by bacteria or fungi.
- Using AzaSite for a long time may cause other bacteria or fungi to grow. If this happens you may get a new infection. Tell your doctor right away if your symptoms do not get better.
What are the possible side effects of AzaSite?
The most common side effect of AzaSite is eye irritation.
Other side effects seen with AzaSite include:
- eye burning, stinging and irritation when the drop hits your eye
- irritation on your eyelid and the skin around your eye
- a feeling of discomfort and irritation or that something is in your eye
- dry eye
- eye pain
- eye itching
- discharge coming from your eye
- changes to the surface of your eye
- blurred vision
- changes in your taste
- hives and rash on your skin
- stuffy nose and sinus infection
- swelling around your eye or of your face
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all of the possible side effects of AzaSite. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store AzaSite?
- Before you open your AzaSite, store it in the refrigerator between 36°F to 46°F (2°C to 8°C).
- After you open your AzaSite, store it at room temperature or the refrigerator between 36°F to 77°F (2°C to 25°C)
- AzaSite should not be stored for more than 14 days after opening. After 14 days, throw the AzaSite bottle away.
- Safely throw away medicine that is out of date or no longer needed.
Keep AzaSite and all medicines out of reach of children.
General information about the safe and effective use of AzaSite
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AzaSite for a condition for which it was not prescribed. Do not give AzaSite to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about AzaSite. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about AzaSite that is written for health professionals.
For more information, call 1-833-838-4028.
What are the ingredients in AzaSite?
Active ingredient: azithromycin
Inactive ingredients: 0.003% benzalkonium chloride, mannitol, citric acid, sodium citrate, poloxamer 407, polycarbophil, edetate disodium (EDTA), sodium chloride, water, and sodium hydroxide.
-
Instructions for Use
AzaSite ® (A-zuh-site)
(azithromycin ophthalmic solution) 1%Read this Instructions for Use for AzaSite before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or treatment.
Important:
- AzaSite is for use as an eye drop only.
The checklist below tells you when to use your medicine for each eye that has bacterial conjunctivitis:
□ □ Day 1:
1 drop in the morning and 1 drop in the evening □ □ Day 2:
1 drop in the morning and 1 drop in the evening □ Day 3
1 drop anytime during the day □ Day 4
1 drop anytime during the day □ Day 5
1 drop anytime during the day □ Day 6
1 drop anytime during the day □ Day 7
1 drop anytime during the day This is a total of 9 drops of AzaSite for each infected eye.
- Avoid letting the applicator tip touch your eye, your fingers, or other objects.
- If a drop misses your eye, try again.
- Follow the steps below to use AzaSite correctly.
Before using a new bottle of AzaSite: 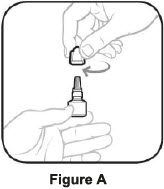
- Turn the white cap clockwise until it comes off. Throw away the white cap. See Figure A
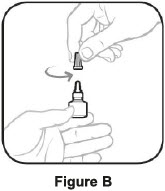
- Hold the bottle straight, turn the tan cap counterclockwise until it comes off. Put the tan cap back on the bottle and close tightly. (This lets out the air.) See Figure B
Wash your hands each time you use AzaSite. To use AzaSite: 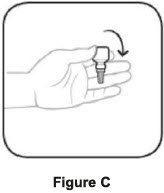
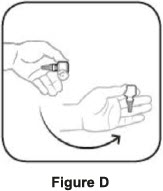
Step 1. Turn the closed bottle upside down. See Figure C Step 2. Shake your hand firmly. This helps move the medicine into the tip of the bottle. See Figure D 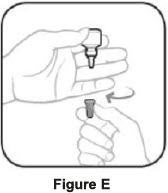
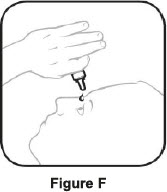
Step 3. Hold the bottle upside down and take off the tan cap. See Figure E Step 4. Tilt your head back. Hold the bottle over your eye and gently squeeze the bottle to let 1 drop into each eye that has bacterial conjunctivitis. Put the tan cap back on the bottle and close tightly. See Figure F If a drop does not come out of the bottle, repeat steps one to four. Manufactured for: Thea Pharma Inc.
Waltham, MA 02451U.S. Patent No.: 7,758,553
AzaSite is a registered trademark of Thea Pharma Inc.
DuraSite is a registered trademark of Sun Pharma.
© 2024. Thea Pharma Inc.
All rights reservedThis Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Revised: 07/2024
-
PRINCIPAL DISPLAY PANEL - 2.5 mL Bottle Carton
NDC: 82584-307-03
AzaSITE ®
(azithromycin ophthalmic solution) 1%Sterile 2.5 mL
Rx Only
FOR OPHTHALMIC USE ONLY
-
INGREDIENTS AND APPEARANCE
AZASITE
azithromycin monohydrate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82584-307 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZITHROMYCIN MONOHYDRATE (UNII: JTE4MNN1MD) (AZITHROMYCIN ANHYDROUS - UNII:J2KLZ20U1M) AZITHROMYCIN ANHYDROUS 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.03 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) POLOXAMER 407 (UNII: TUF2IVW3M2) POLYCARBOPHIL (UNII: W25LM17A4W) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82584-307-02 1 in 1 CARTON 11/01/2022 1 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 82584-307-03 1 in 1 CARTON 11/01/2022 2 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050810 11/01/2022 Labeler - Thea Pharma Inc. (117787029) Establishment Name Address ID/FEI Business Operations Woodstock Sterile Solutions, Inc. 117895702 manufacture(82584-307) , label(82584-307) , pack(82584-307) , analysis(82584-307)
Trademark Results [AZASITE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AZASITE 78426374 3345071 Live/Registered |
INSITE VISION INCORPORATED 2004-05-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.