AnYi Beauty Bum Bum Cream
AnYi Beauty Bum Bum Cream by
Drug Labeling and Warnings
AnYi Beauty Bum Bum Cream by is a Otc medication manufactured, distributed, or labeled by Guangzhou Zhicunxiu Cosmetics Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
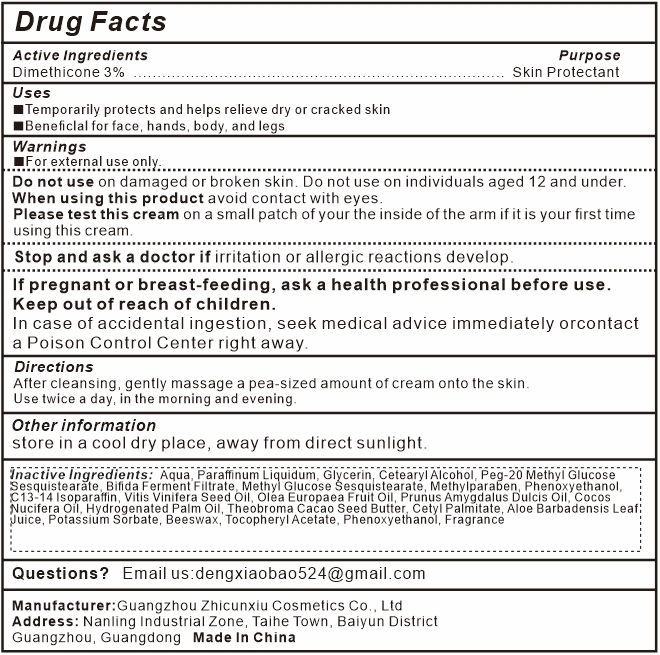
ANYI BEAUTY BUM BUM CREAM- dimethicone cream
Guangzhou Zhicunxiu Cosmetics Co., Ltd
----------
AnYi Beauty Bum Bum Cream
Uses
- Temporarily protects and helps relieve dry or cracked skin
- Beneficlal for face, hands, body, and legs
Warnings
For external use only.
Do not useon damaged or broken skin. Do not use on individuals aged 12 and under.
When using this productavoid contact with eyes.
Please test this creamon a small patch of your the inside of the arm if it is your first time using this crea m.
Stop and ask a doctor ifirritation or allergic reactions develop.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of accidental ingestion, seek medical advice immediately orcontact a Poison Control Center right away.
Directions
After cleansing, gently massage a pea-sized amount of cream onto the skin. Use twice a day, in the morning and evening.
Inactive Ingredients
Aqua, Paraffinum Liquidum, Glycerin, Cetearyl Alcohol, Peg-20 Methyl Glucose Sesquistearate, Dimethicone, Bifida Ferment Filtrate, Methyl Glucose Sesquistearate, Methylparaben, Phenoxyethanol, C13-14 Isoparaffin, Vitis Vinifera Seed Oil, Olea Europaea Fruit Oil, Prunus Amygdalus Dulcis Oil, Cocos Nucifera Oil, Hydrogenated Palm Oil, Theobroma Cacao Seed Butter, Cetyl Palmitate, Aloe Barbadensis Leaf Juice, Potassium Sorbate, Beeswax, Tocopheryl Acetate, Phenoxyethanol, Fragrance
| ANYI BEAUTY BUM BUM CREAM
dimethicone cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Guangzhou Zhicunxiu Cosmetics Co., Ltd (402779051) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Zhicunxiu Cosmetics Co., Ltd | 402779051 | manufacture(83867-0045) | |