LiquiCal with Magnesium Liquid Multivitamin
LiquiCal with Magnesium by
Drug Labeling and Warnings
LiquiCal with Magnesium by is a Prescription medication manufactured, distributed, or labeled by PureTek Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LIQUICAL WITH MAGNESIUM- multivitamin liquid
PureTek Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
LiquiCal with Magnesium
Liquid Multivitamin
DRUG DESCRIPTION:
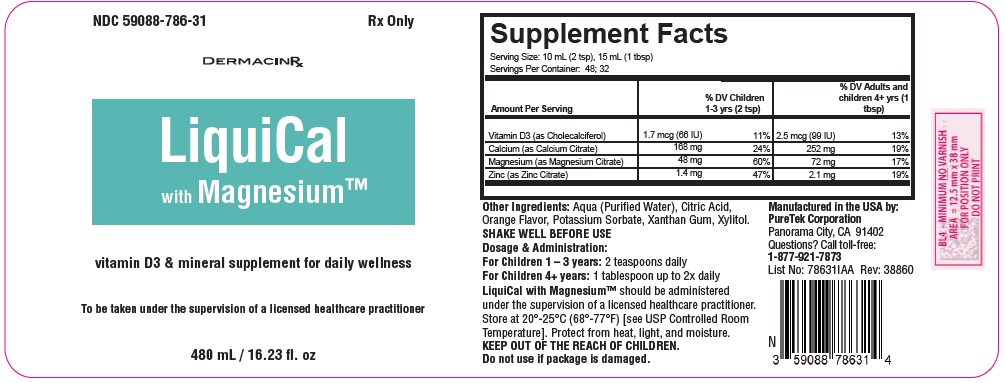
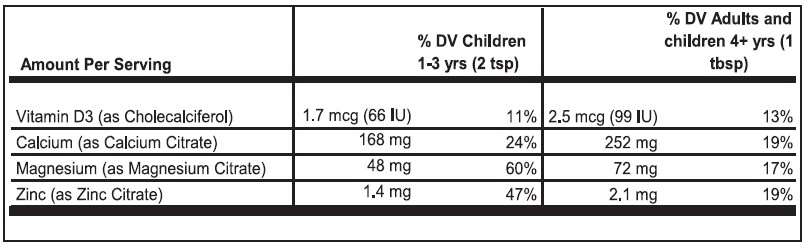
Vitamin D3 (Cholecalciferol): Vitamin D3 plays a pivotal role in maintaining bone health and may also help mitigate mood disturbances. It is recommended to seek a supplement offering 600-800 IU of Vitamin D3 daily, although personal dosing should be discussed with a licensed healthcare practitioner.
Calcium (Calcium Citrate): Calcium is a key mineral that fortifies bones, particularly crucial during menopause due to heightened osteoporosis risks. Women over 50 typically require around 1000-1300 mg of calcium daily, although intake should be calibrated based on diet.
Magnesium (Magnesium Citrate): Magnesium offers relief from muscle cramps, mood fluctuations, and sleep disturbances.
Zinc Citrate: Zinc is an essential trace element that has a critical role in maintaining structural and catalytic functions of >200 enzymes involved in major metabolic pathways, including nucleic acid metabolism, protein synthesis, and cell division.
OTHER INGREDIENTS:
Aqua (Purified Water), Citric Acid, Orange Flavor, Potassium Sorbate, Xanthan Gum, Xylitol.
INDICATIONS AND USAGE:
LiquiCal with Magnesium™ is indicated to provide support in children's healthy bone growth and development.
CONTRAINDICATIONS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. LiquiCal with Magnesium™ is contraindicated in patients with hypercalcemia, malabsorption syndrome, abnormal sensitivity to the toxic effects of vitamin D, and hypervitaminosis D.
WARNINGS AND PRECAUTIONS:
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breastfeeding. Consult your doctor.
ADVERSE REACTIONS:
A number of adverse events are possibly influenced by calcium supplementation; these include myocardial infarction, constipation, colorectal neoplasms, and kidney stone.
Among the side effects of magnesium citrate that are more frequently reported are headaches, nausea, vomiting, diarrhea, constipation, stomach cramps, decreased appetite, muscle tremor, tingling, and numbness.
You should call your doctor for medical advice about serious adverse events. To report adverse side effects or to obtain product information, contact PureTek Corporation, at 1-877-921-7873.
DOSAGE AND ADMINISTRATION:
Shake well before use.
For Children 1 – 3 years: 2 teaspoons daily
For Adult and Children 4+ years: 1 tablespoon up to 2x daily
LiquiCal with Magnesium™ should be administered under the supervision of a licensed healthcare practitioner.
| LIQUICAL WITH MAGNESIUM
multivitamin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - PureTek Corporation (785961046) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.