DEXMEDETOMIDINE HYDROCHLORIDE injection, solution, concentrate DEXMEDETOMIDINE HYDROCHLORIDE injection
Dexmedetomidine in Dextrose by
Drug Labeling and Warnings
Dexmedetomidine in Dextrose by is a Prescription medication manufactured, distributed, or labeled by WG Critical Care, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEXMEDETOMIDINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for DEXMEDETOMIDINE HYDROCHLORIDE INJECTION.
DEXMEDETOMIDINE HYDROCHLORIDE injection, for intravenous use
Initial U.S. Approval: 1999RECENT MAJOR CHANGES
-
Indications and Usage, Intensive Care Unit Sedation (1.1) 09/2016
Dosage and Administration, Recommended Dosage (2.2)
and Dosage Modifications in Geriatric Patients (2.3) 09/2016
Warnings and Precautions, Withdrawal Adverse Reactions (5.5) 09/2016
INDICATIONS AND USAGE
Dexmedetomidine Hydrochloride Injection is a central alpha-2 adrenergic agonist indicated for: (1)
- Sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting. Administer Dexmedetomidine Hydrochloride Injection by continuous infusion not to exceed 24 hours. (1.1)
- Sedation of non-intubated patients prior to and/or during surgical and other procedures. (1.2)
DOSAGE AND ADMINISTRATION
- Dilute in 0.9% Sodium Chloride Injection to concentration of 4 mcg/mL prior to administration. (2.1, 2.6)
- The 200 mcg/50 mL and 400 mcg/100 mL single-dose bag, do not require further dilution prior to administration. (2.6)
- To be administered only by health care providers skilled in management of patients in the intensive care or operating room setting. (2.1)
- Administer intravenously using a controlled infusion device. (2.1)
- Administration duration should not exceed 24 hours. (2.1)
- Continuously monitor blood pressure, heart rate, and oxygen levels during administration and as clinically appropriate after discontinuation. (2.1)
Initiation of Intensive Care Unit Sedation (2.2) (2)
Procedure (2)
Recommended Loading Infusion Dosage (2)
ICU Sedation (2)
1 mcg/kg over 10 minutes (2)
Maintenance of Intensive Care Unit Sedation (2.2) (2)
Procedure (2)
Recommended Maintenance Infusion Dosage (2)
Maintenance (2)
0.2 to 0.7 mcg/kg/hour. (2)
Initiation of Procedural Sedation (2.2) (2)
Procedure (2)
Recommended Loading Infusion Dosage (2)
More invasive procedures or awake fiberoptic intubation (2)
1 mcg/kg over 10 minutes (2)
Less invasive procedures such as ophthalmic surgery (2)
0.5 mcg/kg over 10 minutes (2)
Maintenance of Procedural Sedation (2.2) (2)
Procedure (2)
Recommended Maintenance Infusion Dosage (2)
- All procedures except awake fiberoptic intubation
Generally, initiate at 0.6 mcg/kg/hour and titrate to achieve desired clinical effect with dosages ranging from 0.2 to 1 mcg/kg/hour. (2)
- Awake fiberoptic intubation
Administer 0.7 mcg/kg/hour until the endotracheal tube is secured (2)
- Geriatric patients (age greater than 65 years): Consider a dose reduction for ICU sedation. Recommended loading infusion dosage for initiation of procedural sedation is 0.5 mcg/kg over 10 minutes. Consider dosage reduction for maintenance of procedural sedation. (2.3, 8.5)
- Hepatic impairment: Consider dosage reduction. (2.4, 8.6)
DOSAGE FORMS AND STRENGTHS
Injection (100 mcg/mL): (3)
- 400 mcg in 4 mL in a multiple-dose vial. (3)
- 1000 mcg in 10 mL in a multiple-dose vial. (3)
- 200 mcg/50 mL single-dose, flexible plastic infusion bag. (3)
- 400 mcg/100 mL single-dose, flexible plastic infusion bag. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Bradycardia and Sinus Arrest: Consider decreasing or stopping dexmedetomidine HCl infusion; decreasing or stopping other medications that depress sinus node function; administering anticholinergic agents (e.g., glycopyrrolate, atropine); and/or administering pressor agents. (5.1)
- Hypotension: Consider decreasing or stopping dexmedetomidine HCl infusion; increasing rate of intravenous fluid administration; elevating lower extremities, and/or administering pressor agents. (5.2)
- Transient Hypertension: Observed primarily during administration of loading dose. Consider reducing loading infusion rate. (5.3)
- Arousability: Patients can become aroused/alert with stimulation; this alone should not be considered as lack of efficacy. (5.4)
- Prolonged exposure to dexmedetomidine beyond 24 hours may be associated with tolerance and tachyphylaxis and a dose-related increase in adverse events. (5.6)
ADVERSE REACTIONS
- The most common adverse reactions in ICU sedation (incidence greater than 2% and greater in patients receiving dexmedetomidine HCl than placebo) were hypotension, nausea, bradycardia, fever, atrial fibrillation and anemia. (6.1)
- The most common adverse reactions in procedural sedation (incidence greater than 2% and greater in patients receiving dexmedetomidine HCl than placebo) were hypotension, respiratory depression, bradycardia, nausea, and dry mouth. (6.1)
- Adverse reactions associated with infusions greater than 24 hours in duration include ARDS, respiratory failure, and agitation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact WG Critical Care at 1-866-562-4708 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
Anesthetics, sedatives/hypnotics, opioids: Can potentiate sedating effects. Consider reducing dosage of dexmedetomidine HCl or co-administered drug. (2.5, 7.1) (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2018
-
Indications and Usage, Intensive Care Unit Sedation (1.1) 09/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Intensive Care Unit Sedation
1.2 Procedural Sedation
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Recommended Dosage
2.3 Dosage Modifications in Geriatric Patients
2.4 Dosage Modifications in Patients with Hepatic Impairment
2.5 Dosage Modifications due to Drug Interactions
2.6 Preparation of Diluted Dexmedetomidine HCl Solution for Administration
2.7 Drug Compatibility
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bradycardia and Sinus Arrest
5.2 Hypotension
5.3 Transient Hypertension
5.4 Arousability
5.5 Withdrawal Adverse Reactions
5.6 Tolerance and Tachyphylaxis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs that Can Potentiate the Sedating Effects of Dexmedetomidine HCl
7.2 Drugs without Clinically Significant Drug Interactions with Dexmedetomidine HCl
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Pharmacology and/or Toxicology
14 CLINICAL STUDIES
14.1 Intensive Care Unit Sedation
14.2 Procedural Sedation
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Intensive Care Unit Sedation
Dexmedetomidine Hydrochloride Injectionis indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting. Dexmedetomidine Hydrochloride Injection should be administered by continuous infusion not to exceed 24 hours.
Dexmedetomidine Hydrochloride Injection has been continuously infused in mechanically ventilated patients prior to extubation, during extubation, and post-extubation. It is not necessary to discontinue Dexmedetomidine Hydrochloride Injection prior to extubation.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- Dexmedetomidine HCl Injection, 400 mcg in 4 mL and 100 mcg in 10 mL vial must be diluted prior to administration. Dexmedetomidine HCl Injection, 200 mcg/50 mL and 400mcg/100mL single-dose bags do not require further dilution prior to administration [see Dosage and Administration (2.6)].
- Dexmedetomidine HCl Injection should be administered only by health care providers skilled in the management of patients in the intensive care or operating room setting.
- Administer by continuous intravenous infusion using a controlled infusion device.
- Administration duration should not exceed 24 hours [see Warnings and Precautions (5.5, 5.6)].
- Continuously monitor blood pressure, heart rate and oxygen levels during the use of Dexmedetomidine HCl Injection and as clinically appropriate after discontinuation.
- Use administration components made with synthetic or coated natural rubber gaskets. Dexmedetomidine HCl Injection has the potential for absorption into some types of natural rubber.
2.2 Recommended Dosage
Dexmedetomidine HCl Injection must be diluted prior to administration [see Dosage and Administration (2.6)]. Table 1 displays the recommended loading and maintenance dosage of Dexmedetomidine HCl Injection in various procedures. Individualize dosages and titrate to desired sedation.
Table 1: Recommended Dosage for Dexmedetomidine HCl Injection Initiation of Intensive Care Unit Sedation
Procedure
Recommended Loading Infusion Dosage
ICU Sedation
1 mcg/kg over 10 minutes
For adult patients being converted from alternate sedative therapy, a loading dose may not be required [see Dosage and Administration (2.2)].
Maintenance of Intensive Care Unit Sedation
Procedure
Recommended Maintenance Infusion Dosage
Maintenance
0.2 to 0.7 mcg/kg/hour.
Adjust the maintenance infusion rate to achieve the targeted level of sedation.
Initiation of Procedural Sedation
Procedure
Recommended Loading Infusion Dosage
For more invasive procedures or for awake fiberoptic intubation
1 mcg/kg over 10 minutes
For less invasive procedures such as ophthalmic surgery
0.5 mcg/kg over 10 minutes
Maintenance of Procedural Sedation
Procedure
Recommended Maintenance Infusion Dosage
- For all procedures except awake fiberoptic intubation
- Generally, initiate the maintenance infusion at 0.6 mcg/kg/hour and titrate to achieve desired clinical effect with dosages ranging from 0.2 mcg/kg/hour to 1 mcg/kg/hour.
- Adjust the maintenance infusion rate to achieve the targeted level of sedation.
- For awake fiberoptic intubation
Administer 0.7 mcg/kg/hour until the endotracheal tube is secured.
2.3 Dosage Modifications in Geriatric Patients
For patients over 65 years of age, for ICU sedation, a dose reduction may be considered. For procedural sedation, the recommended intravenous loading infusion dosage of Dexmedetomidine HCl Injection for initiation of procedural sedation is 0.5 mcg/kg infused over 10 minutes. Consider dosage reduction for maintenance of procedural sedation [see Use in Specific Populations (8.5)].
2.4 Dosage Modifications in Patients with Hepatic Impairment
In patients with hepatic impairment, consider dosage reduction of Dexmedetomidine HCl Injection [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.5 Dosage Modifications due to Drug Interactions
When co-administered with anesthetics, sedatives/hypnotics, or opioids, consider dosage reduction of Dexmedetomidine HCl Injection [see Drug Interactions (7.1)].
2.6 Preparation of Diluted Dexmedetomidine HCl Solution for Administration
Dexmedetomidine HCL Injection, 400 mcg in 4 mL and 1000 mcg in 10 mL (100 mcg/mL)
Dexmedetomidine HCl Injection must be diluted prior to administration to a final concentration of 4 mcg/mL by adding:
- 2 mL of Dexmedetomidine HCl Injection to 48 mL of 0.9% Sodium Chloride Injection to a total volume of 50 mL or
- 4 mL of Dexmedetomidine HCl Injection to 96 mL of 0.9% Sodium Chloride Injection to a total of volume of 100 mL
Gently shake and mix well. Prior to administration, visually inspect the diluted dexmedetomidine HCl solution for particulate matter and discoloration (the diluted solution should be a clear, colorless solution).
Prior to use, may store the diluted dexmedetomidine HCl solution for up to 4 hours at room temperature or up to 24 hours at 2o to 8oC.
Discard unused portion.
Dexmedetomidine HCL Injection, 200 mcg/50 mL and 400 mcg/100 mL (4 mcg/mL) flexible plastic infusion bag
Dexmedetomidine HCL Injection is supplied in a flexible plastic infusion bag containing a premixed, ready-to-use dexmedetomidine solution in 5% dextrose in water. No further dilution of these preparations are necessary.
2.7 Drug Compatibility
Diluted dexmedetomidine HCl solution for administration is compatible with and may be co-administered with:
- 0.9% Sodium Chloride in Water Injection
- 5% Dextrose in Water Injection
- Mannitol Injection (20%)
- Lactated Ringer’s Injection
- Magnesium Sulfate Injection (100 mg/mL)
- Potassium Chloride Injection (0.3%)
Diluted dexmedetomidine HCl solution is not compatible for co-administration through the same intravenous catheter with:
- Amphotericin B or diazepam
- Blood or plasma because physical compatibility has not been established.
-
3 DOSAGE FORMS AND STRENGTHS
Dexmedetomidine Hydrochloride Injection is clear and colorless, and is available in a 100 mcg/mL (vial) and 4 mcg/mL (bag) strength as follows:
- 400 mcg in 4 mL in a multiple-dose glass vial
- 1000 mcg in 10 mL in a multiple-dose glass vial
- 200 mcg/50 mL single-dose, flexible plastic infusion bag
- 400 mcg/100 mL single-dose, flexible plastic infusion bag
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Bradycardia and Sinus Arrest
Bradycardia and sinus arrest have been reported following administration of dexmedetomidine HCl to young, healthy adult volunteers with high vagal tone or following rapid intravenous or bolus administration of dexmedetomidine HCl. Bradycardia has also been reported in association with intravenous infusion of dexmedetomidine HCl. Some of these cases have resulted in fatalities. Dexmedetomidine HCl decreases sympathetic nervous system activity and has the potential to augment bradycardia induced by vagal stimuli. Elderly patients and patients with advanced heart block, severe ventricular dysfunction, hypovolemia, diabetes mellitus, and/or chronic hypertension are at increased risk of bradycardia following administration of dexmedetomidine HCl. Closely monitor heart rate and other hemodynamic parameters during administration of dexmedetomidine HCl. In patients who develop bradycardia, consider decreasing or stopping the dexmedetomidine HCl infusion; decreasing or stopping other medications that depress the sinus node function; administering anticholinergic agents (e.g., glycopyrrolate, atropine) to modify vagal tone; and/or administering pressor agents. In patients with significant cardiovascular dysfunction, more advanced resuscitative measures may be required.
5.2 Hypotension
Hypotension has been reported in association with intravenous infusion of dexmedetomidine HCl. Some of these cases have resulted in fatalities. Elderly patients [see Use in Specific Populations (8.5)] and patients with advanced heart block, severe ventricular dysfunction, hypovolemia, diabetes mellitus, and/or chronic hypertension are at increased risk of hypotension following administration of dexmedetomidine HCl. Closely monitor blood pressure and other hemodynamic parameters during administration of dexmedetomidine HCl. If hypotension occurs, consider decreasing or stopping the dexmedetomidine HCl infusion; increasing the rate of intravenous fluid administration; elevating the lower extremities; and/or administering pressor agents.
5.3 Transient Hypertension
Transient hypertension has been observed primarily during administration of the dexmedetomideine HCl loading dose and is likely due to the initial peripheral vasoconstrictive effects of dexmedetomidine . If treatment of the transient hypertension is necessary, consider reducing the loading infusion rate.
5.4 Arousability
Some patients receiving dexmedetomidine HCl have been observed to be arousable and alert when stimulated. This alone should not be considered as evidence of lack of efficacy in the absence of other clinical signs and symptoms.
5.5 Withdrawal Adverse Reactions
Intensive Care Unit Sedation
With administration up to 7 days, regardless of dose, 12 (5%) dexmedetomidine HCl adult subjects experienced at least 1 event related to withdrawal within the first 24 hours after discontinuing study drug and 7 (3%) dexmedetomidine HCl adult subjects experienced at least 1 event 24 to 48 hours after end of study drug. The most common events were nausea, vomiting, and agitation.
In adult subjects, tachycardia and hypertension requiring intervention in the 48 hours following study drug discontinuation occurred at frequencies of <5%. If tachycardia and/or hypertension occurs after discontinuation of dexmedetomidine HCl supportive therapy is indicated.
Procedural Sedation
In adult subjects, withdrawal symptoms were not seen after discontinuation of infusions of dexmedetomidine HCl less than 6 hours in duration.
5.6 Tolerance and Tachyphylaxis
Use of dexmedetomidine HCl beyond 24 hours has been associated with tolerance (reduction in response after longer duration; a higher dosage of dexmedetomidine HCl is required to produce the same effect that was obtained at a lower dosage); tachyphylaxis (a sudden decrease in response); and a dosage-related increase in adverse reactions. Administration duration should not exceed 24 hours [see Dosage and Administration (2.1)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Bradycardia and sinus arrest [see Warnings and Precautions (5.1)]
- Hypotension [see Warnings and Precautions (5.2)]
- Transient hypertension [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Intensive Care Unit Sedation
Adverse reaction information is derived from the continuous infusion trials of dexmedetomidine HCl for sedation in the Intensive Care Unit setting in which 1007 adult patients received dexmedetomidine HCl. The mean total dose was 7.4 mcg/kg (range: 0.8 to 84.1), mean dose per hour was 0.5 mcg/kg/hr (range: 0.1 to 6.0) and the mean duration of infusion of 15.9 hours (range: 0.2 to 157.2). The population was between 17 to 88 years of age, 43% ≥65 years of age, 77% male and 93% Caucasian. Treatment-emergent adverse reactions occurring at an incidence of >2% are provided in Table 2.
Table 2: Adverse Reactions with an Incidence >2%— Adult Intensive Care Unit Sedation Population <24 hours*
Adverse Event All Dexmedetomidine HCl
(N = 1007)
(%)Randomized Dexmedetomidine HCl
(N = 798)
(%)Placebo
(N = 400)
(%)Propofol
(N = 188)
(%)Hypotension
25%
24%
12%
13%
Hypertension
12%
13%
19%
4%
Nausea
9%
9%
9%
11%
Bradycardia
5%
5%
3%
0
Atrial Fibrillation
4%
5%
3%
7%
Pyrexia
4%
4%
4%
4%
Dry Mouth
4%
3%
1%
1%
Vomiting
3%
3%
5%
3%
Hypovolemia
3%
3%
2%
5%
Atelectasis
3%
3%
3%
6%
Pleural Effusion
2%
2%
1%
6%
Agitation
2%
2%
3%
1%
Tachycardia
2%
2%
4%
1%
Hyperthermia
2%
2%
3%
0
Chills
2%
2%
3%
2%
Hyperglycemia
2%
2%
2%
3%
Hypoxia
2%
2%
2%
3%
Post-procedural Hemorrhage
2%
2%
3%
4%
Pulmonary Edema
1%
1%
1%
3%
Ventricular Tachycardia
<1%
1%
1%
5%
*26 subjects in the all dexmedetomidine HCl group and 10 subjects in the randomized dexmedetomidine HCl group had exposure for greater than 24 hours.
Adverse reaction information was also derived from the placebo-controlled, continuous infusion trials of dexmedetomidine HCl for sedation in the surgical intensive care unit setting in which 387 adult patients received dexmedetomidine HCl for less than 24 hours.
Table 3: Treatment-Emergent Adverse Events Occurring in >1% Of All Dexmedetomidine-Treated Adult Patients and at an Incidence Greater than Placebo in the Randomized Placebo-Controlled Continuous Infusion <24 Hours ICU Sedation Studies
Adverse Event
Randomized Dexmedetomidine
(N = 387)Placebo
(N = 379)Hypotension
28%
13%
Nausea
11%
9%
Bradycardia
7%
3%
Fever
5%
4%
Atrial Fibrillation
4%
3%
Anemia
3%
2%
Dry Mouth
3%
1%
Pleural Effusion
2%
1%
Oliguria
2%
<1%
Thirst
2%
<1%
In a controlled clinical trial, dexmedetomidine HCl was compared to midazolam for ICU sedation exceeding 24 hours duration in adult patients. Key treatment emergent adverse events occurring in dexmedetomidine or midazolam treated patients in the randomized active comparator continuous infusion long-term intensive care unit sedation study are provided in Table 4. The number (%) of subjects who had a dose-related increase in treatment-emergent adverse events by maintenance adjusted dose rate range in the dexmedetomidine HCl group is provided in Table 5.
Table 4: Key Treatment-Emergent Adverse Events Occurring in Dexmedetomidine- or Midazolam-Treated Adult Patients in the Randomized Active Comparator Continuous Infusion Long-Term Intensive Care Unit Sedation Study
Adverse Event
Dexmedetomidine
(N = 244)Midazolam
(N = 122)Hypotension requiring intervention1
28%
27%
Bradycardia2
42%
19%
Bradycardia requiring intervention
5%
1%
Pyrexia
7%
2%
Agitation
7%
6%
Hyperglycemia
7%
2%
Respiratory Failure
5%
3%
Renal Failure Acute
2%
1%
Acute Respiratory Distress Syndrome
2%
1%
1 Hypotension was defined in absolute terms as Systolic blood pressure of <80 mmHg or Diastolic blood pressure of <50 mmHg or in relative terms as ≤30% lower than pre-study drug infusion value.
2 Bradycardia was defined in absolute terms as <40 bpm or in relative terms as ≤30% lower than pre-study drug infusion value.
The following adverse events occurred between 2 and 5% for dexmedetomidine HCl and Midazolam, respectively: renal failure acute (2.5%, 0.8%), acute respiratory distress syndrome (2.5%, 0.8%), and respiratory failure (4.5%, 3.3%).
Table 5. Number (%) of Adult Subjects Who Had a Dose-Related Increase in Treatment Emergent Adverse Events by Maintenance Adjusted Dose Rate Range in the Dexmedetomidine HCl Group
Dexmedetomidine HCl mcg/kg/hr
Adverse Event
≤0.7*
(N = 95)>0.7 to ≤1.1*
(N = 78)>1.1*
(N = 71)Constipation
6%
5%
14%
Agitation
5%
8%
14%
Anxiety
5%
5%
9%
Edema Peripheral
3%
5%
7%
Atrial Fibrillation
2%
4%
9%
Respiratory Failure
2%
6%
10%
Acute Respiratory Distress Syndrome
1%
3%
9%
*Average maintenance dose over the entire study drug administration
Procedural Sedation
Adverse reaction information is derived from the two trials for procedural sedation in which 318 adult patients received dexmedetomidine HCl (Studies 1 and 2) [see Clinical Studies (14.2)]. The mean total dose was 1.6 mcg/kg (range: 0.5 mcg/kg to 6.7 mcg/kg), mean dosage per hour was 1.3 mcg/kg/hour (range: 0.3 mcg/kg/hour to 6.1 mcg/kg/hour) and the mean duration of infusion was 1.5 hours (range: 0.1 hour to 6.2 hours). The population was between 18 to 93 years of age, 30% greater than or equal to 65 years of age, 52% male and 61% Caucasian.
Adverse reactions that occurred at an incidence of greater than 2% of patients receiving dexmedetomidine HCl and at an incidence greater than placebo are provided in Table 6. Pre-specified criteria for the vital signs to be reported as adverse reactions are footnoted below the table. The decrease in respiratory rate and hypoxia was similar between dexmedetomidine HCl and comparator groups in both studies.
Table 6: Adverse Reactions* in Clinical Trials of Dexmedetomidine HCl for Adult Procedural Sedation
Adverse Reaction Dexmedetomidine HCl
(N = 318)
(%)Placebo
(N = 113)
(%)Hypotension1
54%
30%
Respiratory Depression2
37%
32%
Bradycardia3
14%
4%
Nausea
3%
2%
Dry Mouth
3%
1%
* Adverse reactions that occurred at an incidence of greater than 2% of patients receiving dexmedetomidine HCl and at an incidence greater than placebo
1 Hypotension was defined in absolute and relative terms as systolic blood pressure of less than 80 mmHg or less than or equal to 30% lower than pre-study drug infusion value, or diastolic blood pressure of less than 50 mmHg.
2 Respiratory depression was defined in absolute and relative terms as respiratory rate (RR) less than 8 beats per minute or greater than 25% decrease from baseline.
3 Bradycardia was defined in absolute and relative terms as less than 40 beats per minute or less than or equal to 30% lower than pre-study drug infusion value.
6.2 Postmarketing Experience
The following adverse reactions, which do not appear elsewhere in this section, have been identified during post-approval use of dexmedetomidine HCl. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypotension and bradycardia were the most common adverse reactions associated with the use of dexmedetomidine HCl during post approval use.
Table 7: Adverse Reactions Experienced During Post-approval Use of Dexmedetomidine HCl Blood and Lymphatic System Disorders
Anemia
Cardiac Disorders
Arrhythmia, atrial fibrillation, atrioventricular block, cardiac arrest, cardiac disorder, extrasystoles, myocardial infarction, supraventricular tachycardia, tachycardia, ventricular arrhythmia, ventricular tachycardia
Eye Disorders
Photopsia, visual impairment
Gastrointestinal Disorders
Abdominal pain, diarrhea, vomiting
General Disorders and Administration Site Conditions
Chills, hyperpyrexia, pain, pyrexia, thirst
Hepatobiliary Disorders
Hepatic function abnormal, hyperbilirubinemia
Investigations
Alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood urea increased, electrocardiogram T wave inversion, gammaglutamyltransferase increased, Electrocardiogram QT prolonged
Metabolism and Nutrition Disorders
Acidosis, hyperkalemia, hypoglycemia, hypovolemia, hypernatremia
Nervous System Disorders
Convulsion, dizziness, headache, neuralgia, neuritis, speech disorder
Psychiatric Disorders
Agitation, confusional state, delirium, hallucination, illusion
Renal and Urinary Disorders
Oliguria, polyuria
Respiratory, Thoracic and Mediastinal Disorders
Apnea, bronchospasm, dyspnea, hypercapnia, hypoventilation, hypoxia, pulmonary congestion, respiratory acidosis
Skin and Subcutaneous Tissue Disorders
Hyperhidrosis, pruritus, rash, urticaria
Surgical and Medical Procedures
Light anesthesia
Vascular Disorders
Blood pressure fluctuation, hemorrhage, hypertension
-
7 DRUG INTERACTIONS
7.1 Drugs that Can Potentiate the Sedating Effects of Dexmedetomidine HCl
Anesthetics (e.g., isoflurane, sevoflurane, propofol), sedatives/hypnotics (e.g., midazolam), and opioids (e.g., alfentanil) can potentiate the sedating effects of dexmedetomidine HCl. Consider reducing the dosage of dexmedetomidine HCl or the co-administered drug.
7.2 Drugs without Clinically Significant Drug Interactions with Dexmedetomidine HCl
Dexmedetomidine HCl had no clinically meaningful effect on the magnitude of neuromuscular blockade associated with rocuronium [see Clinical Pharmacology (12.2)].
In clinical trials where other vasodilators or negative chronotropic agents were co-administered with dexmedetomidine HCl an additive hypotensive or bradycardic effect was not observed. Nonetheless, close monitoring of hemodynamic parameters (e.g., blood pressure, heart rate) is recommended if other vasodilators or negative chronotropic agents are co-administered with dexmedetomidine HCl.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies conducted with dexmedetomidine hydrochloride in pregnant women to inform any drug-associated risks. A published in vitro human placenta study reported placental transfer of dexmedetomidine hydrochloride. Rats subcutaneously administered dexmedetomidine HCl during organogenesis showed pregnancy loss and reduced live pups at doses equivalent to the maximum recommended human dose (MRHD). Reduced fetal weights were observed in rats administered subcutaneously dexmedetomidine HCl at a dose that is less than one-half of the MRHD during gestation and lactation. In this study, elevated fetal and embryocidal toxicity and delayed motor development were observed in second generation offspring. No fetal malformations were observed in animal reproduction studies with subcutaneous administration of dexmedetomidine HCl during organogenesis in rats and rabbits at doses approximately equal to and one-half the MRHD, respectively [see Data]. The background risk in the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Animal Data
Teratogenic effects were not observed in rats following subcutaneous administration of dexmedetomidine HCl during the period of fetal organogenesis (from gestation day 5 to 16) with doses up to 200 mcg/kg (representing a dose approximately equal to the MRHD based on body surface area) or in rabbits following intravenous administration of dexmedetomidine HCl during the period of fetal organogenesis (from gestation day 6 to 18) with doses up to 96 mcg/kg (representing approximately half the human exposure at the MRHD based on plasma area under the time-curve comparison). However, fetal toxicity, as evidenced by increased post-implantation losses and reduced live pups, was observed in rats at a subcutaneous dose of 200 mcg/kg. The no-effect dose in rats was 20 mcg/kg (representing a dose less than the MRHD based on a body surface area comparison). In another reproductive toxicity study when dexmedetomidine HCl was administered subcutaneously to pregnant rats at 8 mcg/kg and 32 mcg/kg (representing a dose less than the MRHD based on a body surface area comparison) from gestation day 16 through weaning, lower offspring weights were observed. Additionally, when offspring of the 32 mcg/kg group were allowed to mate, elevated fetal and embryocidal toxicity and delayed motor development was observed in second generation offspring.
8.2 Lactation
Risk Summary
There is no information regarding the presence of dexmedetomidine hydrochloride in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Radio-labeled dexmedetomidine administered subcutaneously to lactating female rats was excreted in milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for dexmedetomidine hydrochloride and any potential adverse effects on the breastfed infant from dexmedetomidine hydrochloride or from the underlying maternal condition.
Clinical Considerations
A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 10 hours (approximately 5 half-lives) after receivingdexmedetomidine hydrochloride in order to minimize potential drug exposure to a breastfed infant.
8.4 Pediatric Use
Safety and efficacy of dexmedetomidine HCl have not been established for Procedural or ICU Sedation in pediatric patients.
8.5 Geriatric Use
Intensive Care Unit Sedation
A total of 729 patients in the clinical studies were 65 years of age and over. A total of 200 patients were 75 years of age and over. In patients greater than 65 years of age, a higher incidence of bradycardia and hypotension was observed following administration of dexmedetomidine HCl [see Warnings and Precautions (5.1, 5.2)]. Therefore a dose reduction may be considered in patients over 65 years of age [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
Procedural Sedation
A total of 131 patients in the procedural sedation clinical studies were 65 years of age and over. A total of 47 patients were 75 years of age and over. Hypotension occurred in a higher incidence in dexmedetomidine HCl-treated patients 65 years or older (72%) and 75 years or older (74%) as compared to patients less than 65 years (47%). In patients greater than 65 years of age, reduce the loading infusion dosage for initiation of procedural sedation and consider reducing the maintenance infusion dosage for maintenance of procedural sedation [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
The dependence potential of dexmedetomidine HCl has not been studied in humans. However, since studies in rodents and primates have demonstrated that dexmedetomidine HCl exhibits pharmacologic actions similar to those of clonidine, it is possible that dexmedetomidine HCl may produce a clonidine-like withdrawal syndrome upon abrupt discontinuation [see Warnings and Precautions (5.5)].
-
10 OVERDOSAGE
Overdosage of dexmedetomidine HCl can cause the adverse reactions generally associated with dexmedetomidine HCl administration [see Warnings and Precautions (5) and Adverse Reactions (6)]. However, these reactions may be more severe. Heart block (e.g., first degree atrioventricular block, second degree heart block) has been reported following overdosage with dexmedetomidine HCl. Cardiac arrest has been reported following loading bolus administration of undiluted Dexmedetomidine HCl Injection.
Dexmedetomidine HCl Injection must be diluted prior to administration [see Dosage and Administration (2.1)]. Management of overdosage should include general supportive measures to sustain the patient through any period of toxicity that may occur.
-
11 DESCRIPTION
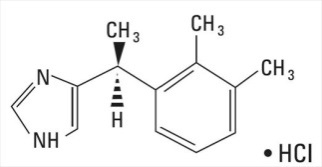
Dexmedetomidine Hydrochloride Injection is a sterile, nonpyrogenic solution suitable for intravenous infusion following dilution. Dexmedetomidine HCl is a central alpha-2 adrenergic agonist. Structurally it is the S-enantiomer of medetomidine and is chemically described as (+)-4-(S)-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole monohydrochloride. Dexmedetomidine HCl has a molecular weight of 236.7 and the empirical formula is C13H16N2 HCl and the structural formula is:
Dexmedetomidine HCl is a white or almost white powder that is freely soluble in water and has a pKa of 7.1. Its partition coefficient in-octanol: water at pH 7.4 is 2.89.
Vials: Dexmedetomidine HCl is supplied as a clear, colorless, isotonic solution with a pH of 4.5 to 7. Each mL contains 118 mcg of dexmedetomidine HCl equivalent to 100 mcg (0.1 mg) of dexmedetomidine, 1.6 mg of methylparaben, 0.2 mg of propylparaben and 9 mg of sodium chloride in water.
Bags: Dexmedetomidine HCl is supplied as a clear, colorless, isotonic solution with a pH of 4.5 to 7. Each mL contains 4.72 mcg of dexmedetomidine HCl equivalent to 4 mcg of dexmedetomidine, 50 mg dextrose monohydrate in water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dexmedetomidine HCl is a central alpha-2 adrenergic agonist with sedative properties. Alpha2 selectivity was observed in animals following slow intravenous infusion of low and medium doses (10 mcg/kg to 300 mcg/kg). Both alpha1 and alpha2 activity was observed following slow intravenous infusion of high doses (greater than or equal to 1000 mcg/kg) or with rapid intravenous administration.
12.2 Pharmacodynamics
In a study in 10 healthy volunteers, respiratory rate and oxygen saturation remained within normal limits and there was no evidence of respiratory depression when dexmedetomidine HCl was administered by intravenous infusion at dosages between 0.2 mcg/kg/hour and 0.7 mcg/kg/hour.
In a study of 10 healthy adult volunteers, administration of dexmedetomidine HCl for 45 minutes at a plasma concentration of 1 ng/mL resulted in no clinically meaningful increases in the magnitude of neuromuscular blockade associated with rocuronium administration.
12.3 Pharmacokinetics
Following intravenous administration, dexmedetomidine exhibited the following pharmacokinetic parameters: a rapid distribution phase with a distribution half-life (t1/2) of approximately 6 minutes; a terminal elimination half-life (t1/2) of approximately 2 hours; and steady-state volume of distribution (Vss) of approximately 118 liters. Clearance was estimated to be approximately 39 L/hour. The mean body weight associated with this clearance estimate was 72 kg.
Dexmedetomidine exhibits linear pharmacokinetics in the dosage range of 0.2 to 0.7 mcg/kg/hr when administered by intravenous infusion for up to 24 hours. Table 8 shows the main pharmacokinetic parameters when dexmedetomidine HCl was infused (after appropriate loading doses) at maintenance infusion rates of 0.17 mcg/kg/hr (target plasma concentration of 0.3 ng/mL) for 12 and 24 hours, 0.33 mcg/kg/hr (target plasma concentration of 0.6 ng/mL) for 24 hours, and 0.70 mcg/kg/hr (target plasma concentration of 1.25 ng/mL) for 24 hours.
Table 8: Mean ± SD Pharmacokinetic Parameters
Parameter
Loading Infusion (min)/Total Infusion Duration (hrs)
10 min/12 hrs
10 min/24 hrs
10 min/24 hrs
35 min/24 hrs
Dexmedetomidine HCL Target Plasma Concentration (ng/mL) and Dose (mcg/kg/hr)
0.3/0.17
0.3/0.17
0.6/0.33
1.25/0.70
t1/2*, hour
1.78 ± 0.30
2.22 ± 0.59
2.23 ± 0.21
2.50 ± 0.61
CL, liter/hour
46.3 ± 8.3
43.1 ± 6.5
35.3 ± 6.8
36.5 ± 7.5
Vss, liter
88.7 ± 22.9
102.4 ± 20.3
93.6 ± 17.0
99.6 ± 17.8
Avg Css #, ng/mL
0.27 ± 0.05
0.27 ± 0.05
0.67 ± 0.10
1.37 ± 0.20
* Presented as harmonic mean and pseudo standard deviation.
# Mean Css = Average steady-state concentration of dexmedetomidine HCl. The mean Css was calculated based on post-dose sampling from 2.5 to 9 hours samples for 12 hour infusion and post-dose sampling from 2.5 to 18 hours for 24 hour infusions.
The loading doses for each of the above indicated groups were 0.5, 0.5, 1 and 2.2 mcg/kg, respectively.
Dexmedetomidine pharmacokinetic parameters after dexmedetomidine HCl maintenance doses of 0.2 to 1.4 mcg/kg/hr for >24 hours were similar to the PK parameters after dexmedetomidine HCL maintenance dosing for < 24 hours in other studies. The values for clearance (CL), volume of distribution (V), and t1/2 were 39.4 L/hr, 152 L, and 2.67 hours, respectively.
Distribution
The steady-state volume of distribution (Vss) of dexmedetomidine was approximately 118 liters. Dexmedetomidine protein binding was assessed in the plasma of normal healthy male and female subjects. The average protein binding was 94% and was constant across the different plasma concentrations tested.
Elimination
The distribution half-life (t1/2) of dexmedetomidine is approximately 6 minutes, the terminal elimination half-life (t1/2) is approximately 2 hours, and clearance is estimated to be approximately 39 L/hour.
Metabolism: Dexmedetomidine undergoes almost complete biotransformation with very little unchanged dexmedetomidine excreted in urine and feces. Biotransformation involves both direct glucuronidation as well as cytochrome P450 mediated metabolism. The major metabolic pathways of dexmedetomidine are: direct N-glucuronidation to inactive metabolites; aliphatic hydroxylation (mediated primarily by CYP2A6) of dexmedetomidine to generate 3-hydroxy-dexmedetomidine, the glucuronide of 3-hydroxy-dexmedetomidine, and 3-carboxy-dexmedetomidine; and N-methylation of dexmedetomidine to generate 3-hydroxy N-methyl-dexmedetomidine, 3-carboxy N-methyl-dexmedetomidine, and dexmedetomidine-N-methyl O-glucuronide.
Excretion: A mass balance study demonstrated that after nine days an average of 95% of the radioactivity, following intravenous administration of radiolabeled dexmedetomidine, was recovered in the urine and 4% in the feces. No unchanged dexmedetomidine was detected in the urine. Approximately 85% of the radioactivity recovered in the urine was excreted within 24 hours after the infusion. Fractionation of the radioactivity excreted in urine demonstrated that products of N-glucuronidation accounted for approximately 34% of the cumulative urinary excretion. In addition, aliphatic hydroxylation of parent drug to form 3-hydroxy-dexmedetomidine, the glucuronide of 3-hydroxy-dexmedetomidine, and 3-carboxylic acid-dexmedetomidine together represented approximately 14% of the dose in urine. N-methylation of dexmedetomidine to form 3-hydroxy N-methyl dexmedetomidine, 3-carboxy N-methyl dexmedetomidine, and N-methyl O-glucuronide dexmedetomidine accounted for approximately 18% of the dose in urine. The N-Methyl metabolite itself was a minor circulating component and was undetected in urine. Approximately 28% of the urinary metabolites have not been identified.
Specific Populations
Age: Geriatric Population: The pharmacokinetic profile of dexmedetomidine HCl was not altered by age. There were no differences in the pharmacokinetics of dexmedetomidine HCl in young (18 to 40 years), middle age (41 to 65 years), and elderly (greater than 65 years) subjects.
Sex: There was no observed difference in dexmedetomidine HCl pharmacokinetics in male and female subjects. Protein binding was similar in males and females.
Hepatic Impairment: In subjects with varying degrees of hepatic impairment (Child-Pugh Class A, B, or C), clearance values for dexmedetomidine HCl were lower than in healthy subjects. The mean clearance values for patients with mild, moderate, and severe hepatic impairment were 74%, 64% and 53% of those observed in the normal healthy subjects, respectively. Mean clearances for free drug were 59%, 51% and 32% of those observed in the normal healthy subjects, respectively [see Dosage and Administration (2.4) and Use in Specific Populations (8.6)].
The fraction of dexmedetomidine HCl that was bound to plasma proteins was significantly decreased in subjects with hepatic impairment compared to subjects with normal hepatic function.
Renal Impairment: Dexmedetomidine HCl pharmacokinetics (Cmax, Tmax, AUC, t1/2, CL, and Vss) were not significantly different in subjects with severe renal impairment (creatinine clearance: less than 30 mL/minute) compared to subjects with normal renal function.
Drug Interaction Studies
In Vitro Studies:In vitro studies in human liver microsomes demonstrated no evidence of cytochrome P450 mediated drug interactions that are likely to be of clinical relevance.
No pharmacokinetic interactions between dexmedetomidine HCl and isoflurane, propofol, alfentanil and midazolam have been demonstrated [see Drug Interactions (7.1)].
Drugs Highly Bound to Plasma Proteins: Dexmedetomidine is highly bound to plasma proteins. The potential for protein binding displacement of dexmedetomidine by other drugs highly bound to proteins (i.e., fentanyl, ketorolac, theophylline, digoxin and lidocaine) was explored in vitro, and negligible changes in the plasma protein binding of dexmedetomidine were observed. The potential for protein binding displacement of other drugs highly bound to proteins (i.e., phenytoin, warfarin, ibuprofen, propranolol, theophylline and digoxin) by dexmedetomidine was explored in vitro and none of these compounds appeared to be significantly displaced by dexmedetomidine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal carcinogenicity studies have not been performed with dexmedetomidine.
Mutagenesis
Dexmedetomidine was not mutagenic in vitro, in either the bacterial reverse mutation assay (E. coli and Salmonella typhimurium) or the mammalian cell forward mutation assay (mouse lymphoma). Dexmedetomidine was clastogenic in the in vitro human lymphocyte chromosome aberration test with, but not without, rat S9 metabolic activation. In contrast, dexmedetomidine was not clastogenic in the in vitro human lymphocyte chromosome aberration test with or without human S9 metabolic activation. Although dexmedetomidine was clastogenic in an in vivo mouse micronucleus test in NMRI mice, there was no evidence of clastogenicity in CD-1 mice.
Impairment of Fertility
Fertility in male or female rats was not affected after daily subcutaneous injections of dexmedetomidine HCl at doses up to 54 mcg/kg (less than the maximum recommended human intravenous dose on a mcg/m2 basis) administered from 10 weeks prior to mating in males, and 3 weeks prior to mating and during mating in females.
13.2 Animal Pharmacology and/or Toxicology
There were no differences in the adrenocorticotropic hormone (ACTH)-stimulated cortisol response in dogs following a single dose of dexmedetomidine compared to saline control. However, after continuous subcutaneous infusions of dexmedetomidine at 3 mcg/kg/hour and 10 mcg/kg/hour for one week in dogs (exposures estimated to be within the clinical range), the ACTH-stimulated cortisol response was diminished by approximately 27% and 40%, respectively, compared to saline-treated control animals indicating a dose-dependent adrenal suppression.
-
14 CLINICAL STUDIES
The safety and efficacy of dexmedetomidine HCl have been evaluated in four randomized, double-blind, placebo-controlled multicenter clinical trials in 1185 adult patients.
14.1 Intensive Care Unit Sedation
Two randomized, double-blind, parallel-group, placebo-controlled multicenter clinical trials included 754 adult patients being treated in a surgical intensive care unit. All patients were initially intubated and received mechanical ventilation. These trials evaluated the sedative properties of dexmedetomidine HCl by comparing the amount of rescue medication (midazolam in one trial and propofol in the second) required to achieve a specified level of sedation (using the standardized Ramsay Sedation Scale) between dexmedetomidine HCl and placebo from onset of treatment to extubation or to a total treatment duration of 24 hours. The Ramsay Level of Sedation Scale is displayed in Table 9.
Table 9: Ramsay Level of Sedation Scale
Clinical Score
Level of Sedation Achieved
6
Asleep, no response
5
Asleep, sluggish response to light glabellar tap or loud auditory stimulus
4
Asleep, but with brisk response to light glabellar tap or loud auditory stimulus
3
Patient responds to commands
2
Patient cooperative, oriented, and tranquil
1
Patient anxious, agitated, or restless
In the first study, 175 adult patients were randomized to receive placebo and 178 to receive dexmedetomidine HCl by intravenous infusion at a dose of 0.4 mcg/kg/hr (with allowed adjustment between 0.2 and 0.7 mcg/kg/hr) following an initial loading infusion of one mcg/kg intravenous over 10 minutes. The study drug infusion rate was adjusted to maintain a Ramsay sedation score of ≥3. Patients were allowed to receive “rescue” midazolam as needed to augment the study drug infusion. In addition, morphine sulfate was administered for pain as needed. The primary outcome measure for this study was the total amount of rescue medication (midazolam) needed to maintain sedation as specified while intubated. Patients randomized to placebo received significantly more midazolam than patients randomized to dexmedetomidine HCl (see Table 10).
A second prospective primary analysis assessed the sedative effects of dexmedetomidine HCl by comparing the percentage of patients who achieved a Ramsay sedation score of ≥3 during intubation without the use of additional rescue medication. A significantly greater percentage of patients in the dexmedetomidine HCl group maintained a Ramsay sedation score of ≥3 without receiving any midazolam rescue compared to the placebo group (see Table 10).
Table 10: Midazolam Use as Rescue Medication During Intubation (ITT) Study One
Placebo
(N = 175)Dexmedetomidine HCl
(N = 178)p-value
Mean Total Dose (mg) of Midazolam
19 mg
5 mg
0.0011*
Standard deviation
53 mg
19 mg
Categorized Midazolam Use
0 mg
43 (25%)
108 (61%)
<0.001**
0–4 mg
34 (19%)
36 (20%)
>4 mg
98 (56%)
34 (19%)
ITT (intent-to-treat) population includes all randomized patients.
* ANOVA model with treatment center.
** Chi-square.
A prospective secondary analysis assessed the dose of morphine sulfate administered to patients in the dexmedetomidine HCl and placebo groups. On average, dexmedetomidine HCl-treated patients received less morphine sulfate for pain than placebo-treated patients (0.47 versus 0.83 mg/h). In addition, 44% (79 of 178 patients) of dexmedetomidine HCl patients received no morphine sulfate for pain versus 19% (33 of 175 patients) in the placebo group.
In a second study, 198 adult patients were randomized to receive placebo and 203 to receive dexmedetomidine HCl by intravenous infusion at a dose of 0.4 mcg/kg/hr (with allowed adjustment between 0.2 and 0.7 mcg/kg/hr) following an initial loading infusion of one mcg/kg intravenous over 10 minutes. The study drug infusion was adjusted to maintain a Ramsay sedation score of ≥3. Patients were allowed to receive “rescue” propofol as needed to augment the study drug infusion. In addition, morphine sulfate was administered as needed for pain. The primary outcome measure for this study was the total amount of rescue medication (propofol) needed to maintain sedation as specified while intubated.
Patients randomized to placebo received significantly more propofol than patients randomized to dexmedetomidine HCl (see Table 11).
A significantly greater percentage of patients in the dexmedetomidine HCl group compared to the placebo group maintained a Ramsay sedation score of ≥3 without receiving any propofol rescue (see Table 11).
Table 11: Propofol Use as Rescue Medication During Intubation (ITT) Study Two
Placebo
(N = 198)Dexmedetomidine HCl
(N = 203)p-value
Mean Total Dose (mg) of Propofol
513 mg
72 mg
<0.0001*
Standard deviation
782 mg
249 mg
Categorized Propofol Use
0 mg
47 (24%)
122 (60%)
<0.001**
0–50 mg
30 (15%)
43 (21%)
>50 mg
121 (61%)
38 (19%)
* ANOVA model with treatment center.
** Chi-square.
A prospective secondary analysis assessed the dose of morphine sulfate administered to patients in the dexmedetomidine HCl and placebo groups. On average, dexmedetomidine HCl-treated patients received less morphine sulfate for pain than placebo-treated patients (0.43 versus 0.89 mg/h). In addition, 41% (83 of 203 patients) of dexmedetomidine HCl patients received no morphine sulfate for pain versus 15% (30 of 198 patients) in the placebo group.
In a controlled clinical trial, dexmedetomidine HCl was compared to midazolam for ICU sedation exceeding 24 hours duration. Dexmedetomidine HCl was not shown to be superior to midazolam for the primary efficacy endpoint, the percent of time patients were adequately sedated (81% versus 81%). In addition, administration of dexmedetomidine HCl for longer than 24 hours was associated with tolerance, tachyphylaxis, and a dose-related increase in adverse events [see Adverse Reactions (6.1)].
14.2 Procedural Sedation
The safety and efficacy of dexmedetomidine HCl for sedation of non-intubated patients prior to and/or during surgical and other procedures were evaluated in two randomized, double-blind, placebo-controlled multicenter clinical trials:
- Study 1 evaluated the sedative properties of dexmedetomidine HCl in patients having a variety of elective surgeries/procedures performed under monitored anesthesia care.
- Study 2 evaluated dexmedetomidine HCl in patients undergoing awake fiberoptic intubation prior to a surgical or diagnostic procedure.
In Study 1, the sedative properties of dexmedetomidine HCl were evaluated by comparing the percent of patients not requiring rescue midazolam to achieve a specified level of sedation using the standardized Observer’s Assessment of Alertness/Sedation Scale (see Table 12).
Table 12: Observer’s Assessment of Alertness/Sedation in Adult Procedural Sedation Study 1 Assessment Categories
Responsiveness
Speech
Facial Expression
Eyes
Composite Score
Responds readily to name spoken in normal tone
Normal
Normal
Clear, no ptosis
5 (alert)
Lethargic response to name spoken in normal tone
Mild slowing or thickening
Mild relaxation
Glazed or mild ptosis (less than half the eye)
4
Responds only after name is called loudly and/or repeatedly
Slurring or prominent slowing
Marked relaxation (slack jaw)
Glazed and marked ptosis (half the eye or more)
3
Responds only after mild prodding or shaking
Few recognizable words
–
–
2
Does not respond to mild prodding or shaking
–
–
–
1 (deep sleep)
Patients were randomized to receive either:
- dexmedetomidine HCl 1 mcg/kg loading dosage given over 10 minutes followed by a maintenance infusion started at 0.6 mcg/kg/hour
- dexmedetomidine HCl 0.5 mcg/kg loading dosage followed by a maintenance infusion started at 0.6 mcg/kg/hour
- placebo (normal saline) loading dosage given over 10 minutes and followed by a placebo maintenance infusion
The maintenance infusion in the two dexmedetomidine groups could be titrated between 0.2 mcg/kg/hour to 1 mcg/kg/hour to achieve the targeted sedation score (Observer’s Assessment of Alertness/Sedation Scale less than or equal to 4). Patients were allowed to receive rescue midazolam as needed to achieve and/or maintain an Observer’s Assessment of Alertness/Sedation Scale less than or equal to 4. After achieving the desired level of sedation, a local or regional anesthetic block was performed. Demographic characteristics were similar between the dexmedetomidine HCl and placebo groups. Efficacy results showed that dexmedetomidine HCl groups were more effective than the placebo group when used to sedate non-intubated patients requiring monitored anesthesia care during surgical and other procedures (see Table 13).
In Study 2, the sedative properties of dexmedetomidine HCl were evaluated by comparing the percent of patients requiring rescue midazolam to achieve or maintain a specified level of sedation using the Ramsay Sedation Scale score more than or equal to 2 (see Table 9). Patients were randomized to receive:
- A loading infusion of dexmedetomidine HCl 1 mcg/kg over 10 minutes followed by a fixed maintenance infusion of 0.7 mcg/kg/hour, or
- A placebo (normal saline) given over 10 minutes followed by a placebo infusion.
After achieving the desired level of sedation, topicalization of the airway occurred. Patients were allowed to receive rescue midazolam as needed to achieve and/or maintain a Ramsay Sedation Scale more than or equal to 2. Demographic characteristics were similar between the dexmedetomidine HCl and comparator groups. For efficacy results see Table 13.
Table 13: Key Efficacy Results of Adult Procedural Sedation Studies (Study 1 and Study 2) Treatment Arm
Number of Patients
Enrolleda% Not Requiring Midazolam Rescue
Confidenceb Interval on the Difference vs. Placebo
Mean (SD) Total Dose of Rescue Midazolam Required
Confidenceb Intervals of the Mean Rescue Dose
- Study 1
- Dexmedetomidine HCl 0.5 mcg/kg (loading) followed by maintenance infusion started at 0.6 mcg/kg/hour
- 134
- 40%
- 37% (27, 48)
- 1.4 (1.7) mg
- -2.7 (-3.4, -2) mg
- Dexmedetomidine HCl 1 mcg/kg (loading) followed by maintenance infusion started at 0.6 mcg/kg/hour
- 129
- 54%
- 51% (40, 62)
- 0.9 (1.5) mg
- -3.1 (-3.8, -2.5) mg
- Placebo
- 63
- 3%
- –
- 4.1 (3) mg
- –
- Study 2
- Dexmedetomidine HCl 1 mcg/kg (loading) followed by a fixed maintenance infusion of 0.7 mcg/kg/hour
- 55
- 53%
- 39% (20, 57)
- 1.1 (1.5) mg
- -1.8 (-2.7, -0.9) mg
- Placebo
- 50
- 14%
- –
- 2.9 (3) mg
- –
SD = Standard deviation
a Based on ITT population defined as all randomized and treated patients.
b Normal approximation to the binomial with continuity correction
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Dexmedetomidine Hydrochloride Injection is clear and colorless, and is available in a 100 mcg/mL strength in clear glass, multiple-dose vials as follows:
NDC No.
Strength
Package
44567-600-04
400 mcg/4 mL
4 vials/carton
44567-601-04
1000 mcg/10 mL
4 vials/carton
Dexmedetomidine Hydrochloride Injection is available in single-dose, single-port, ready-to-use flexible plastic infusion bags in a foil laminate overwrap as follows:
NDC No.
Strength
Package
44567-602-24
200 mcg/50 mL*
24 bags/carton
44567-603-24
400 mcg/100 mL
24 bags/carton
*Partial fill container 50 mL volume in 100 mL container.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. It is recommended that the infusion bags be kept in the overwrap until ready to use. PROTECT INFUSION BAGS FROM FREEZING.
-
17 PATIENT COUNSELING INFORMATION
Advise patients, their families, or caregivers to report to their health care provider symptoms that occur within 48 hours after the administration of Dexmedetomidine HCl Injection such as:
- Nervousness, agitation, and headaches which may be associated with an infusion lasting for more than 6 hours
- Weakness, confusion, excessive sweating, weight loss, abdominal pain, salt cravings, diarrhea, constipation, dizziness or light-headedness
Important Potential Adverse Reactions Following Drug Discontinuation
Advise the patient, their families, or caregivers to contact their health care provider if they develop any of the following symptoms within 48 hours of receiving Dexmedetomidine HCl Injection: weakness, confusion, excessive sweating, weight loss, abdominal pain, salt cravings, diarrhea, constipation, dizziness or light-headedness.
Manufactured for:
WG Critical Care, LLC
Paramus, NJ 07652
Vials made in Finland
Bags made in Switzerland
Revised: July 2018
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
- NDC: 44567-601-04
Dexmedetomidine HCl Injection
1000 mcg/10 mL (100 mcg/mL)
Rx only WG Critical Care
-
Package/Label Display Panel
NDC: 44567-602-24 Rx only
Dexmedetomidine
HCl Injection
200 mcg/50 mL (4 mcg/mL)
For Intravenous Use Only
-
Package/Label Display Panel
NDC: 44567-603-24 Rx only
Dexmedetomidine
HCl Injection
400 mcg/100 mL (4 mcg/mL)
For Intravenous Use Only
-
INGREDIENTS AND APPEARANCE
DEXMEDETOMIDINE HYDROCHLORIDE
dexmedetomidine hydrochloride injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 44567-600 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMEDETOMIDINE HYDROCHLORIDE (UNII: 1018WH7F9I) (DEXMEDETOMIDINE - UNII:67VB76HONO) DEXMEDETOMIDINE 100 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44567-600-04 4 in 1 CARTON 01/26/2017 1 4 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206628 04/19/2016 DEXMEDETOMIDINE HYDROCHLORIDE
dexmedetomidine hydrochloride injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 44567-601 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMEDETOMIDINE HYDROCHLORIDE (UNII: 1018WH7F9I) (DEXMEDETOMIDINE - UNII:67VB76HONO) DEXMEDETOMIDINE 100 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44567-601-04 4 in 1 CARTON 01/26/2017 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206628 04/19/2016 DEXMEDETOMIDINE HYDROCHLORIDE
dexmedetomidine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 44567-602 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMEDETOMIDINE HYDROCHLORIDE (UNII: 1018WH7F9I) (DEXMEDETOMIDINE - UNII:67VB76HONO) DEXMEDETOMIDINE 4 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44567-602-24 24 in 1 CARTON 10/18/2018 1 50 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206628 04/19/2016 DEXMEDETOMIDINE HYDROCHLORIDE
dexmedetomidine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 44567-603 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMEDETOMIDINE HYDROCHLORIDE (UNII: 1018WH7F9I) (DEXMEDETOMIDINE - UNII:67VB76HONO) DEXMEDETOMIDINE 4 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44567-603-24 24 in 1 CARTON 10/18/2018 1 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206628 04/19/2016 Labeler - WG Critical Care, LLC (829274633)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.