Orajel Dry Mouth Moisturizing Gel
Orajel Dry Mouth Moistruizing by
Drug Labeling and Warnings
Orajel Dry Mouth Moistruizing by is a Otc medication manufactured, distributed, or labeled by Church & Dwight Co., Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ORAJEL DRY MOUTH MOISTRUIZING- glycerin gel
Church & Dwight Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
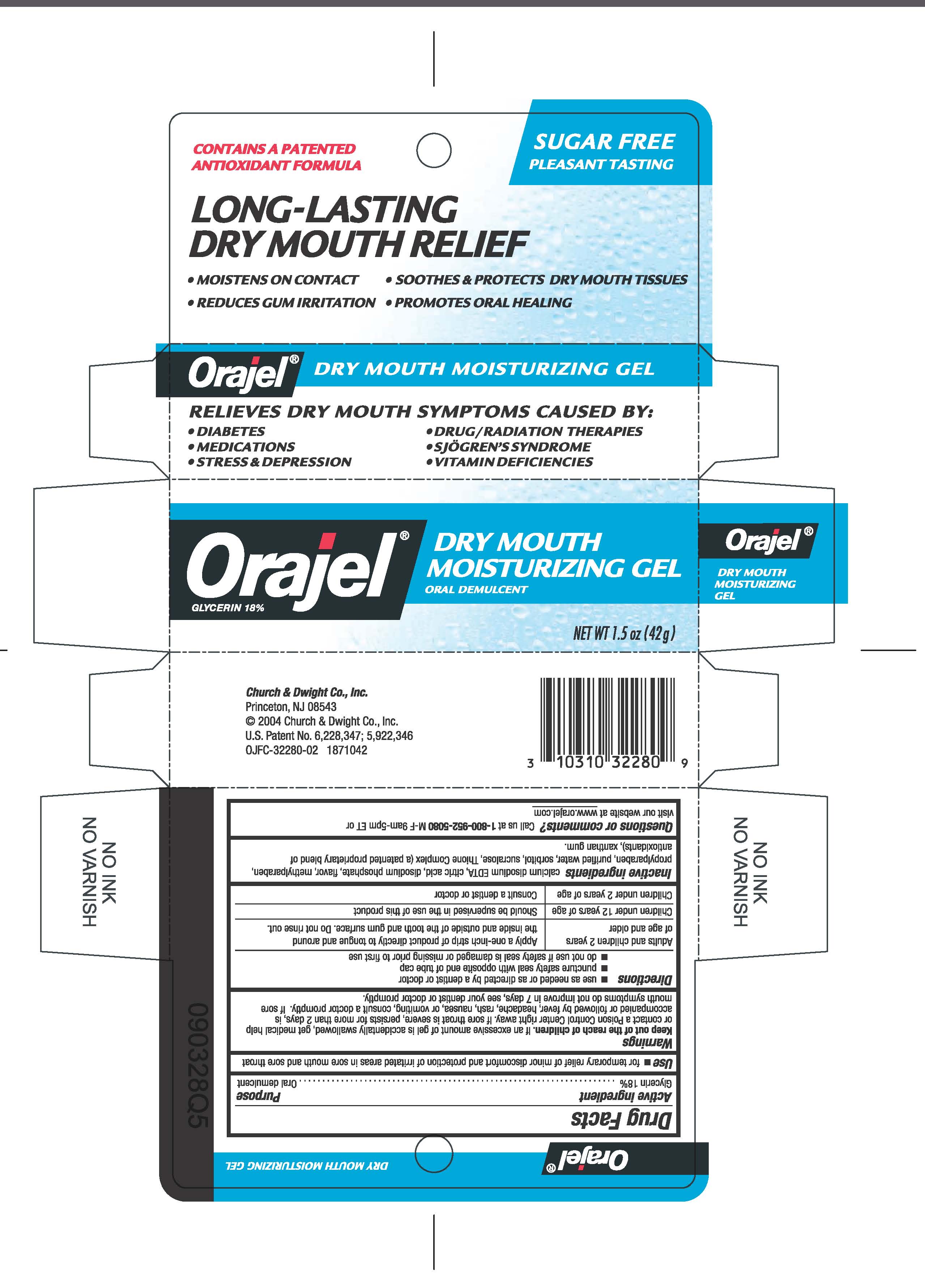
Orajel Dry Mouth Moisturizing Gel
Directions
- use as needed or as directed by a dentist or doctor
- puncture safety seal with opposite end of tube cap
- do not use if safety seal is damaged or mission prior to first use
Adults and children 2 years of age and older
- Apply a one-inch strip of product directly to tongue and around the inside and outside of the tooth and gum surface. Do not rinse out.
Children under 12 years of age
- Should be supervised in the use of this product
Children under 2 years of age
- Consult a dentist or doctor
Warnings
Keep out of the reach of children. If an excessive amount of gel is accidentally swallowed, get medical help or contact a Poison Control Center right away. If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, see your dentist or doctor promptly.
Inactive ingredients: calcium disodium EDTA, citric acid, disodium phosphate, flavor, methylparaben, propylparaben, purified water, sorbitol, sucralose, Thinone Complex (a patented proprietarey blend of antioxidants, xanthan gum.
Questions or comments: Call us at 1-800952-5080 M-F 9am-5pm ET or visit our webiste at www.orajel.com
Use: for the temporary relief of minor discomfort and protection of irritated areas in sore mouth and sore throat
Warnings
Keep out of reach of children. If an excessive amount of gel is accidentally swallowed, get medical help or contact a Poison Control Center right away. If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, see your dentist or doctor promptly.
CONTAINS A PATENTED
ANTIOXIDANT FORMULA
SUGAR FREE
PLEASANT TASTING
LONG-LASTING
DRY MOUTH RELIEF
- MOISTENS ON CONTACT
- REDUCES GUM IRRITATION
- SOOTHES AND PROTECTS DRY MOUTH TISSUES
- PROMOTES ORAL HEALING
Orajel DRY MOUTH MOISTURIZING GEL
RELIEVES DRY MOUTH SYMPTOMS CAUSED BY:
- DIABETES
- MEDICATIONS
- STRESS AND DEPRESSION
- DRUG/RADIATION THERAPIES
- SJOGRENS SYNDROME
- VITAMIN DEFICIENCIES
Orajel
GLYCERIN 18%
DRY MOUTH
MOISTURIZING GEL
ORAL DEMULCENT
NET WT 1.5 oz (42 g)
| ORAJEL DRY MOUTH MOISTRUIZING
glycerin gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Church & Dwight Co., Inc. (001211952) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Church & Dwight Co., Inc. | 043690812 | manufacture(10237-719) | |