TRONOLANE- pramoxine hcl cream
Tronolane by
Drug Labeling and Warnings
Tronolane by is a Otc medication manufactured, distributed, or labeled by New Genesis/Monticello Drug LLC, ULTRAtab Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only. Certain persons can develop allergic reactions to ingredients in this product. If symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase, discontinue use and consult a doctor.
-
Directions
- Adults and children 12 years of age and older: Apply externally to the affected area up to 5 times daily.
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly or cleanse by patting or blotting with an appropriate cleansing pad.
- Gently dry by patting or blotting with toilet tissue before application of this product.
- To use applicator: Remove cap from tube and attach applicator. Gently squeeze tube to apply: do not insert applicator into rectum. Thoroughly cleanse applicator after use.
- Children under 12 years of age: consult a doctor.
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
NDC: 11868-390-48
Tronolane®
Anesthetic Cream for HemorrhoidsDUAL-ACTION
FORMULARapid Relief
of Pain & ItchingOdor-free Non-greasy Non-staining
1 OZ (28 g)
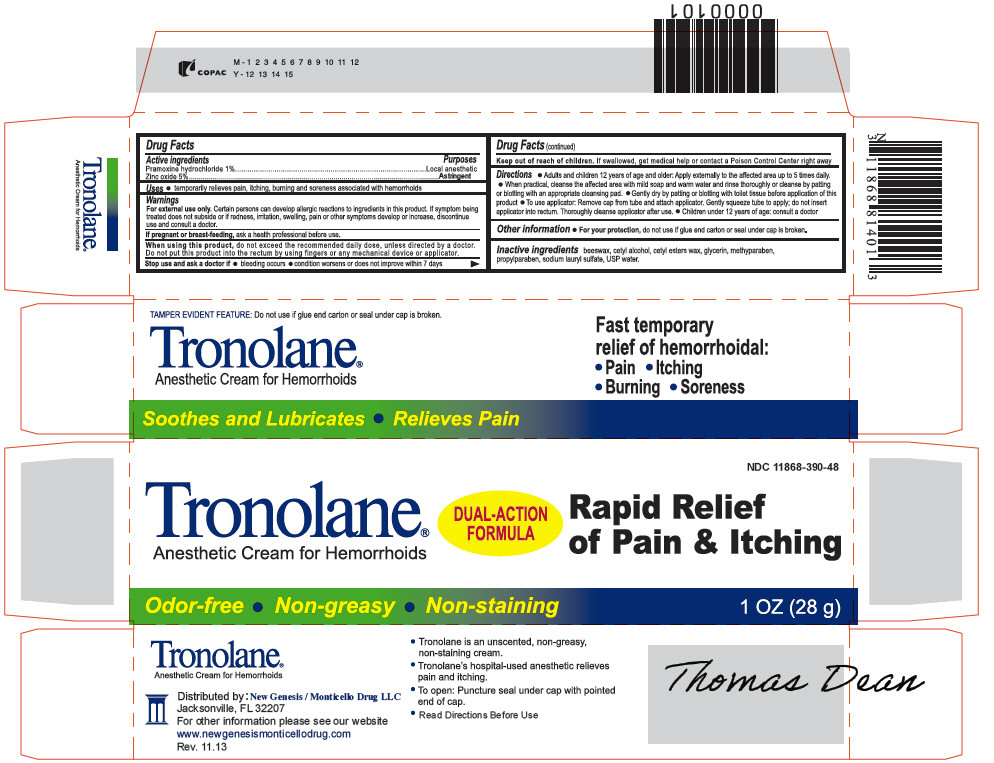
-
INGREDIENTS AND APPEARANCE
TRONOLANE
pramoxine hcl creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11868-390 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 50 mg in 1 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ALCOHOL (UNII: 936JST6JCN) CETYL ESTERS WAX (UNII: D072FFP9GU) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11868-390-48 1 in 1 CARTON 12/04/2011 1 NDC: 11868-390-28 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 11868-390-59 1 in 1 CARTON 03/04/2015 2 NDC: 11868-390-58 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 12/04/2011 Labeler - New Genesis/Monticello Drug LLC (079396165) Registrant - ULTRAtab Laboratories, Inc. (151051757) Establishment Name Address ID/FEI Business Operations ULTRAtab Laboratories, Inc. 151051757 manufacture(11868-390)
Trademark Results [Tronolane]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRONOLANE 86665145 4907878 Live/Registered |
New Genesis/Monticello Drug, LLC 2015-06-17 |
 TRONOLANE 86238203 4628603 Live/Registered |
Monticello Drug Company 2014-04-01 |
 TRONOLANE 73282245 1200811 Dead/Cancelled |
Abbott Laboratories 1980-10-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.