These highlights do not include all the information needed to use MEGESTROL ACETATE ORAL SUSPENSION safely and effectively. See full prescribing information for MEGESTROL ACETATE ORAL SUSPENSION. MEGESTROL ACETATE oral suspension Initial U.S. Approval: 1993
Megestrol Acetate by
Drug Labeling and Warnings
Megestrol Acetate by is a Prescription medication manufactured, distributed, or labeled by Bora Pharmaceutical Laboratories Inc., BORA PHARMACEUTICAL LABORATORIES INC., TWi Pharmaceuticals, Inc. Zhongli Plant, TWi Pharmaceuticals, Inc. Zhongli Plant Building II. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MEGESTROL ACETATE- megestrol acetate suspension
Bora Pharmaceutical Laboratories Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MEGESTROL ACETATE ORAL SUSPENSION safely and effectively. See full prescribing information for MEGESTROL ACETATE ORAL SUSPENSION.
MEGESTROL ACETATE oral suspension Initial U.S. Approval: 1993 RECENT MAJOR CHANGESINDICATIONS AND USAGEMegestrol acetate oral suspension is a progestin indicated for the treatment of anorexia, cachexia, or an unexplained significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome (AIDS) (1). DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSOral suspension containing 125 mg of megestrol acetate per mL (3). CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse events occurring in > 5% of all patients receiving 800mg/20mL of megestrol acetate oral suspension in the two clinical efficacy trials were nausea, diarrhea, impotence, rash, flatulence, hypertension, and asthenia (6.2). To report SUSPECTED ADVERSE REACTIONS, contact TWi Pharmaceuticals, Inc. at 1-844-518-2989 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2023 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Megestrol acetate oral suspension is indicated for the treatment of anorexia, cachexia, or an unexplained significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome (AIDS).
Limitations of Use
Therapy with megestrol acetate oral suspension for weight loss should only be instituted after treatable causes of weight loss are sought and addressed. These treatable causes include possible malignancies, systemic infections, gastrointestinal disorders affecting absorption, endocrine disease, renal disease or psychiatric diseases.
Megestrol acetate oral suspension is not intended for prophylactic use to avoid weight loss.
2 DOSAGE AND ADMINISTRATION
2.2 Dosing and Administration
- The recommended adult initial dosage of megestrol acetate oral suspension is 625 mg/day (5 mL/day or one teaspoon daily).
- Shake the container well before using.
- This strength (125 mg/mL) is not substitutable with other strengths (e.g., 40 mg/mL). Refer to the prescribing information of the 40 mg/mL product for dosage recommendations for the 40 mg/mL strength.
3 DOSAGE FORMS AND STRENGTHS
Megestrol acetate oral suspension is milky white, lemon flavored, and contains 125 mg per mL.
5 WARNINGS AND PRECAUTIONS
5.1 General
- Effects on HIV viral replication have not been determined.
- Use with caution in patients with a history of thromboembolic disease.
5.2 Fetal Toxicity
Based on animal studies, megestrol acetate may cause fetal harm when administered to a pregnant woman. Pregnant rats treated with low doses of megestrol acetate resulted in a reduction in fetal weight and number of live births, and feminization of male fetuses. There are no available human data to assess for any drug associated risks of miscarriage, birth defects, or adverse maternal or fetal outcomes. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, advise the patient of the potential hazard to the fetus [see Use in Specific Populations ( 8.1), Nonclinical Toxicology ( 13.1)].
Obtain a pregnancy test in females of reproductive potential prior to initiating treatment with megestrol acetate oral suspension [see Dosage and Administration ( 2.1)]. Advise females of reproductive potential to use effective contraception while taking megestrol acetate oral suspension [see Use in Specific Populations ( 8.3)].
5.3 Adrenal Insufficiency
The glucocorticoid activity of megestrol acetate oral suspension has not been fully evaluated. Clinical cases of overt Cushing's Syndrome have been reported in association with the chronic use of megestrol acetate. In addition, clinical cases of adrenal insufficiency have been observed in patients receiving or being withdrawn from chronic megestrol acetate therapy in the stressed and non-stressed state. Furthermore, adrenocorticotropin (ACTH) stimulation testing has revealed the frequent occurrence of asymptomatic pituitary-adrenal suppression in patients treated with chronic megestrol acetate therapy. Therefore, the possibility of adrenal insufficiency should be considered in any patient receiving or being withdrawn from chronic megestrol acetate oral suspension therapy who presents with symptoms and/or signs suggestive of hypoadrenalism (e.g., hypotension, nausea, vomiting, dizziness, or weakness) in either the stressed or non-stressed state. Laboratory evaluation for adrenal insufficiency and consideration of replacement or stress doses of a rapidly acting glucocorticoid are strongly recommended in such patients. Failure to recognize inhibition of the hypothalamic-pituitary adrenal axis may result in death. Finally, in patients who are receiving or being withdrawn from chronic megestrol acetate oral suspension therapy, consideration should be given to the use of empiric therapy with stress doses of a rapidly acting glucocorticoid during stress or serious intercurrent illness (e.g., surgery, infection).
6 ADVERSE REACTIONS
6.1 Serious and Otherwise Important Adverse Reactions
The following serious reactions and otherwise important adverse drug reactions are discussed in greater detail in other sections of the labeling:
6.2 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of megestrol acetate oral suspension, 125 mg/mL was based on three studies of megestrol acetate oral suspension (40 mg/mL). The adverse reaction profile of these 3 studies are presented below.
Adverse events which occurred in at least 5% of patients in any arm of the two clinical efficacy trials and the open trial for megestrol acetate oral suspension are listed below by treatment group. All patients listed had at least one post baseline visit during the 12 study weeks.
|
Percentage of Patients Reporting Adverse Events |
|||||||
|
Trial 1 (N=236) |
Trial 2 (N=87) |
Open Label Trial |
|||||
|
Placebo |
Placebo | ||||||
|
Megestrol Acetate mg/day |
0 |
100 |
400 |
800 |
0 |
800 |
1200 |
|
No. of Patients |
N=34 |
N=68 |
N=69 |
N=65 |
N=38 |
N=49 |
N=176 |
|
Diarrhea |
15 |
13 |
8 |
15 |
8 |
6 |
10 |
|
Impotence |
3 |
4 |
6 |
14 |
0 |
4 |
7 |
|
Rash |
9 |
9 |
4 |
12 |
3 |
2 |
6 |
|
Flatulence |
9 |
0 |
1 |
9 |
3 |
10 |
6 |
|
Hypertension |
0 |
0 |
0 |
8 |
0 |
0 |
4 |
|
Asthenia |
3 |
2 |
3 |
6 |
8 |
4 |
5 |
|
Insomnia |
0 |
3 |
4 |
6 |
0 |
0 |
1 |
|
Nausea |
9 |
4 |
0 |
5 |
3 |
4 |
5 |
|
Anemia |
6 |
3 |
3 |
5 |
0 |
0 |
0 |
|
Fever |
3 |
6 |
4 |
5 |
3 |
2 |
1 |
|
Libido Decreased |
3 |
4 |
0 |
5 |
0 |
2 |
1 |
|
Dyspepsia |
0 |
0 |
3 |
3 |
5 |
4 |
2 |
|
Hyperglycemia |
3 |
0 |
6 |
3 |
0 |
0 |
3 |
|
Headache |
6 |
10 |
1 |
3 |
3 |
0 |
3 |
|
Pain |
6 |
0 |
0 |
2 |
5 |
6 |
4 |
|
Vomiting |
9 |
3 |
0 |
2 |
3 |
6 |
4 |
|
Pneumonia |
6 |
2 |
0 |
2 |
3 |
0 |
1 |
|
Urinary Frequency |
0 |
0 |
1 |
2 |
5 |
2 |
1 |
Adverse events which occurred in 1% to 3% of all patients enrolled in the two clinical efficacy trials with at least one follow-up visit during the first 12 weeks of the study are listed below by body system. Adverse events occurring less than 1% are not included. There were no significant differences between incidence of these events in patients treated with megestrol acetate and patients treated with placebo.
Body as a Whole -abdominal pain, chest pain, infection, moniliasis and sarcoma
Cardiovascular System -cardiomyopathy and palpitation
Digestive System -constipation, dry mouth, hepatomegaly, increased salivation and oral moniliasis
Hemic and Lymphatic System -leukopenia
Metabolic and Nutritional -LDH increased, edema and peripheral edema
Nervous System -paresthesia, confusion, convulsion, depression, neuropathy, hypesthesia and abnormal thinking
Respiratory System -dyspnea, cough, pharyngitis and lung disorder
Skin and Appendages -alopecia, herpes, pruritus, vesiculobullous rash, sweating and skin disorder
Special Senses -amblyopia
Urogenital System -albuminuria, urinary incontinence, urinary tract infection and gynecomastia.
7 DRUG INTERACTIONS
7.1 Indinavir
Due to the significant decrease in the exposure of indinavir by megestrol acetate, administration of a higher dose of indinavir should be considered when coadministering with megestrol acetate [See Clinical Pharmacology ( 12.3)] .
7.2 Zidovudine and Rifabutin
No dosage adjustment for zidovudine and rifabutin is needed when megestrol acetate is coadministered with these drugs [See Clinical Pharmacology ( 12.3)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data, megestrol acetate may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy [see Contraindications ( 4)] . There are no available human data to assess for any drug-associated risks of miscarriage, birth defects, or adverse maternal or fetal outcomes. There are no adequate animal developmental toxicity data at clinically relevant doses. Pregnant rats treated with low doses of megestrol acetate resulted in a reduction in fetal weight and number of live births, and feminization of male fetuses at doses below maximum recommended clinical dosing based on body surface area ( see Data). Advise a pregnant women of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Reproduction studies were performed in pregnant rats at oral doses ranging from 0.05 to 12.5 mg/kg/day, which are below the maximum recommended clinical dose based on body surface area. Reduction in fetal weight and number of live births were observed at 12.5 mg/kg/day (5 times lower than the maximum clinical dose) when dams were dosed on days 12 through 18 of pregnancy. Feminization of male fetuses also occurred when dams were dosed on days 13 through 20 of pregnancy at 3 mg/kg/day, approximately 22 times below the maximum clinical dose.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1. Megestrol acetate is present in human milk. There are no data on the effects of megesterol acetate on the breastfed infant or the effects on milk production. Because of the potential for HIV transmission and adverse effects on a breastfed infant, instruct mothers not to breastfeed if they are taking megestrol acetate oral suspension.
8.3 Females and Males of Reproductive Potential
Pregnancy testing
Pregnancy testing is recommended prior to treatment with megestrol acetate oral suspension [see Dosage and Administration ( 2.1), Use in Specific Populations ( 8.1)].
Contraception
Megestrol acetate oral suspension may cause fetal harm when administered during pregnancy [see Use in Specific Populations ( 8.1)]. Advise females of reproductive potential to use effective contraception during treatment with megestrol acetate oral suspension.
8.5 Geriatric Use
Clinical studies of megestrol acetate oral suspension in the treatment of anorexia, cachexia, or an unexplained significant weight loss in patients with AIDS did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Megestrol acetate is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
10 OVERDOSAGE
No serious unexpected side effects have resulted from studies involving megestrol acetate oral suspension administered in dosages as high as 1200 mg/day. In post-marketing experience, limited reports of overdose have been received. Signs and symptoms reported in the context of overdose included diarrhea, nausea, abdominal pain, shortness of breath, cough, unsteady gait, listlessness, and chest pain. There is no specific antidote for overdose with megestrol acetate oral suspension. In case of overdose, appropriate supportive measures should be taken. Megestrol acetate has not been tested for dialyzability; however, due to its low solubility it is postulated that dialysis would not be an effective means of treating overdose.
11 DESCRIPTION
Megestrol acetate oral suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-Hydroxy-6-methyl pregna-4,6-diene-3,20-dione acetate. Solubility at 37° C in water is 2 mcg per mL, solubility in plasma is 24 mcg per mL. Its molecular weight is 384.52.
The chemical formula is C 24H 32O 4and the structural formula is represented as follows:
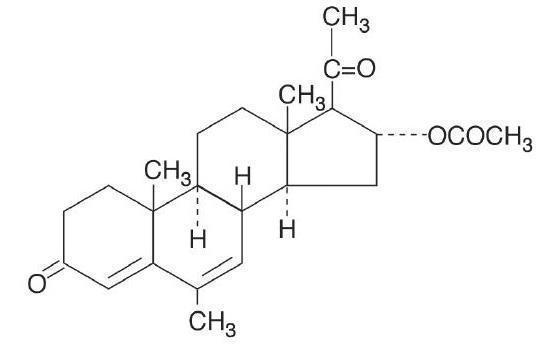
Megestrol acetate oral suspension is an oral suspension containing 125 mg of megestrol acetate per mL.
Megestrol acetate oral suspension contains the following inactive ingredients: alcohol (max 0.07% v/v from flavor), citric acid monohydrate, hypromellose, lemon flavor, sodium benzoate, sodium citrate dihydrate, sodium lauryl sulfate, and sucrose.
The USP dissolution test is pending.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Several investigators have reported on the appetite enhancing property of megestrol acetate and its possible use in cachexia. The precise mechanism by which megestrol acetate produces effects in anorexia and cachexia is unknown at the present time.
12.3 Pharmacokinetics
Absorption and Distribution
Mean plasma concentrations of megestrol acetate after administration of 625 mg (125 mg/mL) of megestrol acetate oral suspension are equivalent under fed conditions to 800 mg (40 mg/mL) of megestrol acetate oral suspension in healthy volunteers.
In order to characterize the dose proportionality of megestrol acetate oral suspension, pharmacokinetic studies across a range of doses were conducted when administered under fasting and fed conditions. Pharmacokinetics of megestrol acetate was linear in the dosing range between 150 mg and 675 mg after megestrol acetate oral suspension administration regardless of meal condition. The mean peak plasma concentration (C max) and the mean area under the concentration time-curve (AUC) after a high fat meal were increased by 48% and 36%, respectively, compared to those under the fasting condition after 625 mg megestrol acetate oral suspension administration. This food effect is less than that seen for the original formulation, megestrol acetate 800 mg/20 mL, where a high fat meal significantly increased AUC and C maxof megestrol acetate to 2-fold and 7-fold, respectively, compared to those under the fasting condition. There was no difference in safety following administration in the fed state, therefore megestrol acetate oral suspension could be taken without regard to meals.
Plasma steady state pharmacokinetics of megestrol acetate was evaluated in 10 adult, cachectic male adult patients with acquired immunodeficiency syndrome (AIDS) and an involuntary weight loss greater than 10% of baseline who received single oral doses of 800 mg/day of megestrol acetate oral suspension for 21 days. The Mean (±1SD) C maxof megestrol acetate was 753 (±539) ng/mL. The mean AUC was 10476 (±7788) ng x hr/mL. Median T maxvalue was five hours.
In another study, 24 asymptomatic HIV seropositive male adult subjects were dosed once daily with 750 mg of megestrol acetate oral suspension for 14 days. Mean C maxand AUC values were 490 (±238) ng/mL and 6779 (±3048) hr x ng/mL, respectively. The median T maxvalue was three hours. The mean C minvalue was 202 (±101) ng/mL. The mean % of fluctuation value was 107 (±40).
Metabolism and Excretion
The major route of drug elimination in humans is urine. When radio-labeled megestrol acetate was administered to humans in doses of 4 to 90 mg, the urinary excretion within 10 days ranged from 56.5% to 78.4% (mean 66.4%) and fecal excretion ranged from 7.7% to 30.3% (mean 19.8%). The total recovered radioactivity varied between 83.1% and 94.7% (mean 86.2%).
Megestrol acetate metabolites which were identified in urine constituted 5% to 8% of the dose administered. Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in urine and feces.
The mean elimination half-life of megestrol ranged from 20 to 50 hours in healthy subjects.
Specific Populations
The pharmacokinetics of megestrol acetate has not been studied in specific population, for example, pediatric, renal impairment, and hepatic impairment.
Drug Interactions
The effects of indinavir, zidovudine or rifabutin on the pharmacokinetics of megestrol acetate were not studied.
Zidovudine
Pharmacokinetic studies show that there are no significant alterations in exposure of zidovudine when megestrol acetate is administered with this drug.
Rifabutin
Pharmacokinetic studies show that there are no significant alterations in exposure of rifabutin when megestrol acetate is administered with this drug.
Indinavir
A pharmacokinetic study in healthy male subjects demonstrated that coadministration of megestrol acetate (675 mg for 14 days) and indinavir (single dose 800 mg) results in a significant decrease in the pharmacokinetic parameters (~32% for C maxand ~21% for AUC) of indinavir.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Data on carcinogenesis were obtained from studies conducted in dogs, monkeys and rats treated with megestrol acetate at doses below the recommended clinical dose based on body surface area. No males were used in the dog and monkey studies. In female beagles, megestrol acetate (0.01, 0.1 or 0.25 mg/kg/day) administered for up to 7 years induced both benign and malignant tumors of the breast at doses greater than or equal to 0.01 mg/kg/day, approximately 75 to 187 times below the maximum clinical dose. In female monkeys, no tumors were found following 10 years of treatment with 0.01, 0.1 or 0.5 mg/kg/day megestrol acetate, up to 65 times below the maximum clinical dose. Pituitary tumors were observed in female rats treated for 2 years with 3.9 or 10 mg/kg/day of megestrol acetate, approximately 6 to 17 times below the maximum clinical dose. The relationship of these tumors in rats and dogs to humans is unknown but should be considered in assessing the risk-to-benefit ratio when prescribing megestrol acetate oral suspension and in surveillance of patients on therapy.
Megestrol acetate induced unscheduled DNA synthesis in primary cultures of human hepatocytes, but not in rat hepatocytes. Megestrol administered to mice increased the frequency of sister chromatid exchange and chromosomal aberrations in bone marrow cells after single intraperitonial doses of 16.25 and 32.50 mg/kg.
Impaired reproductive capability was observed in male offspring born to female rats treated during gestation days 13 through 20 with oral doses greater than or equal to 3 mg/kg/day megestrol, approximately 22 times below the maximum clinical dose. Female dogs treated daily with megestrol oral capsules for 7 years experienced a complete cessation of estrus activity and ovulation at doses of 0.1, or 0.25 mg/kg/day, approximately 187 and 75 times below the maximum clinical dose, respectively.
13.2 Animal Pharmacology and/or Toxicology
Long-term treatment with megestrol acetate oral suspension may increase the risk of respiratory infections. A trend toward increased frequency of respiratory infections, decreased lymphocyte counts and increased neutrophil counts was observed in a two-year chronic toxicity/carcinogenicity study of megestrol acetate conducted in rats.
14 CLINICAL STUDIES
The efficacy of megestrol acetate oral suspension, 125 mg/mL, was based on two trials of megestrol acetate oral suspension (40 mg/mL). These two trials are described below.
Trial 1
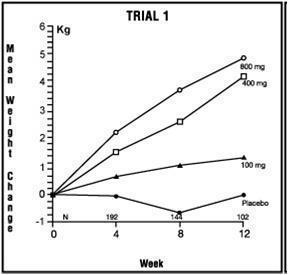
One was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate (MA) at doses of 100 mg, 400 mg, and 800 mg per day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 270 patients entered on study, 195 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12 week period or had one post baseline weight measurement but dropped out for therapeutic failure. The percent of patients gaining 2.3 kg or more at maximum weight gain in 12 study weeks was statistically significantly greater for the 800 mg (64%) and 400 mg (57%) MA-treated groups than for the placebo group (24%). Mean weight increased from baseline to last evaluation in 12 study weeks in the 800 mg MA-treated group by 3.5 kg, the 400 mg MA group by 1.9 kg, the 100 mg MA group by 0.9 kg and decreased in the placebo group by 0.7 kg. Mean weight changes at 4, 8 and 12 weeks for patients evaluable for efficacy in the two clinical trials is shown graphically. Changes in body composition during the 12 study weeks as measured by bioelectrical impedance analysis showed increases in non-water body weight in the MA-treated groups. In addition, edema developed or worsened in only 3 patients.
Greater percentages of MA-treated patients in the 800 mg group (89%), the 400 mg group (68%) and the 100 mg group (72%), than in the placebo group (50%), showed an improvement in appetite at last evaluation during the 12 study weeks. A statistically significant difference was observed between the 800 mg MA-treated group and the placebo group in the change in caloric intake from baseline to time of maximum weight change. Patients were asked to assess weight change, appetite, appearance, and overall perception of well-being in a 9 question survey. At maximum weight change only the 800 mg MA-treated group gave responses that were statistically significantly more favorable to all questions when compared to the placebo-treated group. A dose response was noted in the survey with positive responses correlating with higher dose for all questions.
Trial 2
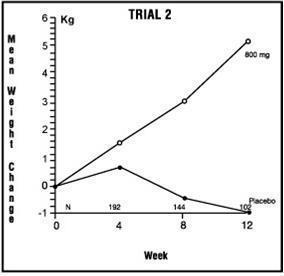
The second trial was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate 800 mg/day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 100 patients entered on study, 65 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12 week period or had one post baseline weight measurement but dropped out for therapeutic failure. Patients in the 800 mg MA-treated group had a statistically significantly larger increase in mean maximum weight change than patients in the placebo group. From baseline to study week 12, mean weight increased by 5.1 kg in the MA-treated group and decreased 1.0 kg in the placebo group. Changes in body composition as measured by bioelectrical impedance analysis showed increases in non-water weight in the MA-treated group ( see Clinical Studies table). No edema was reported in the MA-treated group. A greater percentage of MA-treated patients (67%) than placebo-treated patients (38%) showed an improvement in appetite at last evaluation during the 12 study weeks; this difference was statistically significant. There were no statistically significant differences between treatment groups in mean caloric change or in daily caloric intake at time to maximum weight change. In the same 9 question survey referenced in the first trial, patients' assessments of weight change, appetite, appearance, and overall perception of well-being showed increases in mean scores in MA-treated patients as compared to the placebo group.
In both trials, patients tolerated the drug well and no statistically significant differences were seen between the treatment groups with regard to laboratory abnormalities, new opportunistic infections, lymphocyte counts, T4 counts, T8 counts, or skin reactivity tests [see Adverse Reactions ( 6)].
Table 2: Megestrol Acetate Oral Suspension
Clinical Efficacy Trials
| Trial 1
Study Accrual Dates 11/88 to 12/90 | Trial 2
Study Accrual Dates 5/89 to 4/91 |
|||||
| Megestrol Acetate, mg/day | 0 | 100 | 400 | 800 | 0 | 800 |
| Entered Patients | 38 | 82 | 75 | 75 | 48 | 52 |
| Evaluable Patients | 28 | 61 | 53 | 53 | 29 | 36 |
| Mean Change in Weight (kg) | ||||||
| Baseline to 12 Weeks | 0.0 | 1.3 | 4.2 | 4..9 | -1.0 | 5.1 |
| % Patients ≥2.3 kg Gain | ||||||
| at Last Evaluation in 12 Weeks | 21 | 44 | 57 | 64 | 28 | 47 |
| Mean Changes in Body Composition*: | ||||||
| Fat Body Mass (kg) | 0.0 | 1.0 | 1.3 | 2.5 | 0.7 | 2.6 |
| Lean Body Mass (kg) | -0.8 | -0.1 | 0.7 | 1.1 | -0.7 | -0.3 |
| Water (liters) | -1.3 | -0.3 | 0.0 | 0.0 | -0.1 | -0.1 |
| % Patients With Improved Appetite: | ||||||
| At Time of Maximum | ||||||
| Weight Change | 50 | 72 | 72 | 93 | 48 | 69 |
| At Last Evaluation in 12 Weeks | 50 | 72 | 68 | 89 | 38 | 67 |
| Mean Change in Daily Caloric Intake: | ||||||
| Baseline to Time of Maximum | ||||||
| Weight Change | -107 | 326 | 308 | 646 | 30 | 464 |
| *Based on bioelectrical impedance analysis determinations at last evaluation in 12 weeks. | ||||||
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Megestrol acetate oral suspension is a milky white, lemon-lime flavored oral suspension containing 125 mg per mL. Available in bottles of 150 mL (5 fl oz).
16.2 Storage
Store megestrol acetate oral suspension between 20° to 25° C (68° to 77° F) and dispense in a tight container. Protect from heat.
16.3 Safe Handling
Health Hazard Data
There is no threshold limit value established by OSHA, NIOSH, or ACGIH. Exposure or overdose at levels approaching recommended dosing levels could result in side effects described above [see Warnings and Precautions ( 5) and Adverse Reactions ( 6)]. Women at risk of pregnancy should avoid such exposure.
17 PATIENT COUNSELING INFORMATION
023The prescriber should inform the patient about the product differences to avoid overdosing or underdosing of megestrol acetate. The recommended adult dosage of megestrol acetate oral suspension is one teaspoon (5 mL) once a day [see Dosage and Administration ( 2)].
Patients using megestrol acetate oral suspension should receive the following instructions:
- This medication is to be used as directed by the physician.
- Megestrol acetate oral suspension (625 mg/5 mL) does not contain the same amount of megestrol acetate as Megace oral suspension or any of the other megestrol acetate oral suspensions. Megestrol acetate oral suspension contains 625 mg of megestrol acetate per 5 mL (125mg/mL) whereas Megace oral suspension and other megestrol acetate oral suspensions contain 800 mg per 20 mL (40 mg/mL).
- Report any adverse reaction experiences while taking this medication.
- Fetal Toxicity
[see Warnings and Precautions (
5.2), Use in Specific Populations (
8.1,
8.3)]
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy.
- Advise female patients of reproductive potential to use effective contraception during treatment with megestrol acetate oral suspension.
- Lactation
[see Use in Specific Populations (
8.2)]
- Advise mothers not to breastfeed because of the risk of passing the HIV-1 virus to the baby in breast milk.
Manufactured for:

TWi Pharmaceuticals USA, Inc.
Paramus, NJ 07652
Manufactured by:
TWi Pharmaceuticals, Inc.
Taoyuan City, 320023, Taiwan
LA-3136-01
Revised: 03/23
Megace® is a registered trademark of Bristol-Myers Squibb Company licensed to Endo Pharmaceuticals Inc.
| MEGESTROL ACETATE
megestrol acetate suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Bora Pharmaceutical Laboratories Inc. (656139511) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BORA PHARMACEUTICAL LABORATORIES INC. | 656139511 | label(43826-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TWi Pharmaceuticals, Inc. Zhongli Plant | 658863394 | manufacture(43826-061) , analysis(43826-061) , pack(43826-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TWi Pharmaceuticals, Inc. Zhongli Plant Building II | 658869462 | manufacture(43826-061) , analysis(43826-061) , pack(43826-061) | |
