Spinosad by ParaPRO LLC / Ferndale Laboratories, Inc. / Pharmaceutics International Inc. SPINOSAD suspension
Spinosad by
Drug Labeling and Warnings
Spinosad by is a Prescription medication manufactured, distributed, or labeled by ParaPRO LLC, Ferndale Laboratories, Inc., Pharmaceutics International Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Spinosad Topical Suspension safely and effectively. See full prescribing information for Spinosad Topical Suspension.
Spinosad Topical Suspension
Initial U.S. Approval: 2011RECENT MAJOR CHANGES
How Supplied (16) 01/2024 INDICATIONS AND USAGE
Spinosad Topical Suspension is a pediculicide indicated for the topical treatment of head lice infestations in adult and pediatric patients 6 months of age and older. (1.1)
Spinosad Topical Suspension is a scabicide indicated for the topical treatment of scabies infestations in adult and pediatric patients 4 years of age and older. (1.2)
DOSAGE AND ADMINISTRATION
- For topical use only. Not for oral, ophthalmic, or intravaginal use. (2)
- Treatment of head lice infestations (2.2):
- Shake bottle well
- Apply a sufficient amount to cover dry scalp, then apply to dry hair
- Rinse off with warm water after 10 minutes
- Repeat treatment only if live lice are seen 7 days after first treatment
- Treatment of scabies infestations (2.3):
- Shake bottle well
- Apply product to skin by rubbing it in to completely cover the body from the neck down to the soles of the feet
- Patients with balding scalp should also apply product to the scalp, hairline, temples, and forehead
- Allow to absorb in the skin and dry for 10 minutes before getting dressed
- Leave on the skin for at least 6 hours before showering or bathing
DOSAGE FORMS AND STRENGTHS
Topical suspension: 0.9% w/w (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Benzyl Alcohol Toxicity: Not recommended in infants below the age of 6 months; potential for increased systemic absorption. (5.1)
ADVERSE REACTIONS
Most common adverse events for lice treatment (>1%) were application site erythema and ocular erythema. (6.1)
Most common adverse reactions for scabies treatment (>1%) were application site irritation (pain and burning) and dry skin. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact ParaPRO LLC at 1-855-628-7622 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION, FDA-approved patient labeling and FDA-approved patient labeling.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Head Lice Infestations
1.2 Scabies Infestations
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Treatment of Head Lice Infestations
2.3 Treatment of Scabies Infestations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Benzyl Alcohol Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Head Lice Infestations
14.2 Scabies Infestations
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Head Lice Infestations
Spinosad Topical Suspension is indicated for the topical treatment of head lice infestations in adult and pediatric patients 6 months of age and older.
Adjunctive Measures for Head Lice Infestations
Spinosad Topical Suspension should be used in the context of an overall lice management program:
- Wash in hot water or dry-clean all recently worn clothing, hats, used bedding and towels.
- Wash personal care items such as combs, brushes and hair clips in hot water.
- A fine-tooth comb or special nit comb may be used to remove dead lice and nits.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- For topical use only. Spinosad Topical Suspension is not for oral, ophthalmic, or intravaginal use.
- Avoid contact with eyes. If Spinosad Topical Suspension gets in or near the eyes, rinse thoroughly with water.
2.2 Treatment of Head Lice Infestations
- Shake bottle well.
- Apply a sufficient amount of Spinosad Topical Suspension to cover dry scalp, then apply to dry hair. Depending on hair length, apply up to 120 mL (one bottle) to adequately cover scalp and hair.
- Leave on for 10 minutes, then thoroughly rinse off with warm water.
- Wash hands after use.
- If live lice are seen 7 days after the first treatment, a second treatment should be applied.
- Apply Spinosad Topical Suspension on pediatric patient only under direct supervision of an adult [see Warnings and Precautions (5.1)].
2.3 Treatment of Scabies Infestations
- Shake bottle well.
- Apply a sufficient amount of Spinosad Topical Suspension to skin to completely cover the body from the neck to the toes (including the soles of the feet).
- For patients with balding scalp, also apply product to the scalp, hairline, temples, and forehead.
- Allow to absorb into the skin and dry for 10 minutes before getting dressed.
- Leave on the skin for at least 6 hours before showering or bathing.
- Apply Spinosad Topical Suspension on pediatric patient only under direct supervision of an adult .
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Benzyl Alcohol Toxicity
Spinosad Topical Suspension contains benzyl alcohol and is not approved for use in neonates and infants below the age of 6 months. Systemic exposure to benzyl alcohol has been associated with serious adverse reactions and death in neonates and low birth-weight infants when administered intravenously [See Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Head Lice Infestations
Spinosad Topical Suspension was studied in two randomized, active-controlled trials (N=552) in subjects with head lice; Table 1 presents selected adverse events, regardless of relationship to Spinosad Topical Suspension, that occurred in at least 1% of subjects.
Table 1: Selected Adverse Events Occurring in at least 1% of Subjects with Head Lice Infestation Signs Spinosad (N=552) Permethrin 1% (N=457) Application site erythema 17 (3%) 31(7%) Ocular erythema 12 (2%) 15 (3%) Application site irritation 5 (1%) 7 (2%) Other less common reactions (less than 1% but more than 0.1%) were application site dryness, application site exfoliation, alopecia, and dry skin.
Scabies Infestations
Spinosad Topical Suspension was studied in three randomized, double-blind, vehicle-controlled trials (Trial 1, Trial 2, and Trial 3) in 592 subjects with scabies infestation, of which 165 were ages 4-17 and 427 were adults. Subjects received a single application of Spinosad Topical Suspension to the skin from the neck to the soles of the feet, which was washed off after a minimum of 6 hours. Table 2 presents adverse reactions related to Spinosad Topical Suspension treatment that occurred in at least 1% of subjects.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Spinosad, the active ingredient in Spinosad Topical Suspension, is not absorbed systemically following topical application, and maternal use is not expected to result in fetal exposure to the drug. Spinosad Topical Suspension contains benzyl alcohol. Topical benzyl alcohol is unlikely to be absorbed through the skin in clinically relevant amounts; therefore, maternal use is not expected to result in fetal exposure to the drug [see Clinical Pharmacology (12.3)].
In animal reproduction studies, no adverse embryofetal effects were seen at oral doses of spinosad up to 200 mg/kg/day in pregnant rats or 50 mg/kg/day in pregnant rabbits administered during the period of organogenesis (see Data). The available data do not allow the calculation of relevant comparisons between the systemic exposure of spinosad in animal studies to the systemic exposure that would be expected in humans after topical use of Spinosad Topical Suspension.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk for birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Systemic embryofetal development studies were conducted in rats and rabbits. Oral doses of 10, 50 and 200 mg/kg/day spinosad were administered during the period of organogenesis (gestational days 6 – 15) to pregnant female rats. No adverse embryofetal effects were noted at any dose. Maternal toxicity occurred at 200 mg/kg/day. Oral doses of 2.5, 10, and 50 mg/kg/day spinosad were administered during the period of organogenesis (gestational days 7 – 19) to pregnant female rabbits. No adverse embryofetal effects were noted at any dose. Maternal toxicity occurred at 50 mg/kg/day.
A two-generation dietary reproduction study was conducted in rats. Oral doses of 3, 10, and 100 mg/kg/day spinosad were administered to male and female rats from 10-12 weeks prior to mating and throughout mating, parturition, and lactation. No reproductive/developmental toxicity was noted at doses up to 10 mg/kg/day. In the presence of maternal toxicity, increased dystocia in parturition, decreased gestation survival, decreased litter size, decreased pup body weight, and decreased neonatal survival occurred at a dose of 100 mg/kg/day.
8.2 Lactation
Risk Summary
Spinosad, the active ingredient in Spinosad Topical Suspension, is not systemically absorbed by the mother following topical application. Therefore, breastfeeding is not expected to result in the exposure of the child to spinosad [see Clinical Pharmacology (12.3)] . Advise breastfeeding women to remove Spinosad Topical Suspension from the breast with soap and water before breastfeeding to avoid direct infant exposure to Spinosad Topical Suspension.
Spinosad Topical Suspension contains benzyl alcohol. Topical benzyl alcohol is unlikely to be absorbed through the skin of breastfeeding women in clinically relevant amounts; therefore, breastfeeding is not expected to result in exposure of the infant to Spinosad Topical Suspension [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Spinosad Topical Suspension and any potential adverse effects on the breastfed child from Spinosad Topical Suspension, or from the underlying maternal condition.
8.4 Pediatric Use
Head Lice Infestation
The safety and effectiveness of Spinosad Topical Suspension for the topical treatment of head lice infestation have been established in pediatric patients 6 months of age and older [see Clinical Pharmacology (12.3) and Clinical Studies (14)] .
Spinosad Topical Suspension is not recommended in pediatric patients below the age of 6 months because of the potential for increased systemic absorption due to a high ratio of skin surface area to body mass and the potential for an immature skin barrier.
Spinosad Topical Suspension contains benzyl alcohol. Intravenous administration of benzyl alcohol has been associated with serious adverse reactions and death in neonates and low birth-weight infants. The "gasping syndrome" (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birthweight infants when administered intravenously. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse.
The minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birthweight infants, as well as patients receiving high dosages of benzyl alcohol, may be more likely to develop toxicity [see Warning and Precautions (5.1)] .
Scabies Infestation
The safety and effectiveness of Spinosad Topical Suspension for the topical treatment of scabies infestation have been established in pediatric patients 4 years of age and older. Use of Spinosad Topical Suspension in this age group is supported by Trial 1 and Trial 2 which included 165 pediatric subjects ages 4 to 17 years old with scabies infestation. The safety and efficacy were generally consistent between pediatric and adult patients. [see Clinical Pharmacology (12.3) and Clinical Studies (14)] .
The safety and effectiveness of Spinosad Topical Suspension have not been established in pediatric patients less than 4 years of age with scabies infestation.
8.5 Geriatric Use
Clinical studies of Spinosad Topical Suspension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Spinosad Topical Suspension is a slightly opaque, light orange-colored, viscous topical suspension.
Spinosad Topical Suspension is a pediculicide and scabicide. Spinosad, the active ingredient, is derived from the fermentation of a soil actinomycete bacterium, Saccharopolyspora spinosa.
Spinosad is a mixture of spinosyn A and spinosyn D in a ratio of approximately 5 to 1 (spinosyn A to spinosyn D).
Spinosyn A: The chemical name is: 1 H-as-Indaceno[3,2-d]oxacyclododecin-7,a5-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2 H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-
Spinosyn D: The chemical name is: 1 H-as-Indaceno[3,2-d]oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy]-13-[[2R,5S,6R)-5-(dimethylamino) tetrahydro-6-methyl-2 H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-4,14-dimethyl-, (2S,3aSR,5aS,5bS,9S,13S,14R,16aS,16bS)-
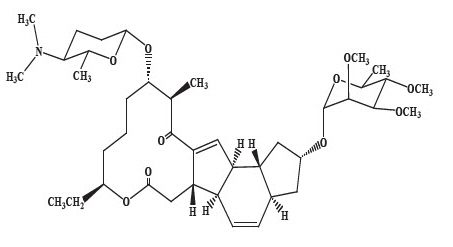
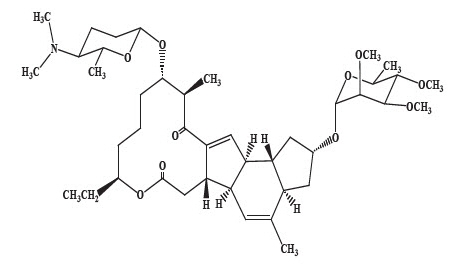
Spinosyn A (C 41H 65NO 10)
MW 731.461Spinosyn D (C 42H 67NO 10)
MW 745.477Spinosad Topical Suspension contains 9 mg spinosad per gram in a vehicle consisting of Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth-20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Spinosad causes neuronal excitation in insects. After periods of hyperexcitation, lice and mites become paralyzed and die.
12.3 Pharmacokinetics
Head Lice Infestations
An open-label, single-center trial was conducted over a period of seven days to determine the pharmacokinetic profile of spinosad 1.8% in pediatric subjects with head lice infestation. Fourteen (14) subjects, 4 – 15 years of age, with head lice were enrolled into the trial. All subjects applied a single topical (scalp) treatment of spinosad 1.8% for 10 minutes, after which the test article was washed off, and subjects underwent plasma sampling. Results demonstrated that spinosad was below the limit of quantitation (3 ng/mL) in all samples. Plasma concentration of benzyl alcohol was not determined in these subjects.
An open-label, two-center trial was conducted over a period of 23 days to determine the pharmacokinetic profile of spinosad 0.9% and the ingredient benzyl alcohol in pediatric subjects with a head lice infestation. Twenty-six (26) subjects between 6 months to 4 years of age were enrolled into the study per protocol. All subjects applied a single topical (scalp) treatment of spinosad 0.9% for 10 minutes, after which the test article was washed off, and subjects underwent plasma sampling over a 12 hour period. Plasma spinosad concentrations were below the limit of quantitation (3 ng/mL) in all samples.
Benzyl alcohol was quantifiable (above 1 μg/mL) in a total of 8 plasma samples in 6 out of 26 subjects (25%): four out of 12 subjects in the 6 months to <2 years age group and two out of 14 subjects in the 2 to 4 years age group. The highest observed concentration was 2.37 μg/mL. Benzyl alcohol concentrations at 12 hours post-treatment were below limit of quantification (1 μg/mL) for all subjects.
Scabies Infestations
An open-label multi-center trial was conducted to determine the pharmacokinetic profile of spinosad 0.9% and the ingredient benzyl alcohol in pediatric subjects with scabies infestation. The PK bioavailability study was completed in 19 pediatrics subjects 5 to 16 years of age. All subjects applied a single topical body treatment of spinosad 0.9% from the neck down to the soles of the feet and allowed treatment to remain on the body for a minimum of 6 hours after which the test article was washed off. Subjects underwent plasma sampling over a 12-hour period after treatment. Plasma spinosad concentrations were below the limit of quantification (3 ng/mL) in all samples.
Benzyl alcohol was quantifiable (above 1 μg/mL) in a total of 9 plasma samples in 6 out of 19 subjects (32%): in 3 out of 10 subjects in the 5 to 9 year age group and in 3 out of 9 subjects in the 10 to 16 years age group. The highest observed concentration was 3.94 μg/mL at 0.5 hours post-treatment but was below limit of quantification at 1 hour post-treatment for one subject in the 10 to 16 years age group. There were two subjects with a benzyl alcohol concentration at 3 hours post-treatment with the highest value of 3.53 μg/mL for one subject in the 5 to 9 years age group. Plasma concentrations were below limit of quantification (1 μg/mL) at 3 hours for all other subjects; no subject had measurable concentrations at the 6 and 12 hour time points. The mean (SD) Cmax, Tmax, and AUC0-12h values for benzyl alcohol were 2.737 (1.107) μg/mL, 1.42 (1.242) hours, and 19.240 μg∙h/mL, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (diet) mouse carcinogenicity study, spinosad was administered to CD-1 mice at doses of 0.0025, 0.008, and 0.036% in the diet (approximately 3.4, 11.4, and 50.9 mg/kg/day for males and 4.2, 13.8, and 67.0 mg/kg/day for females) for 18 months. No treatment-related tumors were noted in the mouse carcinogenicity study up to the highest doses evaluated in this study of 50.9 mg/kg/day in male mice and 13.8 mg/kg/day in female mice. Female mice treated with a dose of 67.0 mg/kg/day were not evaluated in this study due to high mortality.
In an oral (diet) rat carcinogenicity study, spinosad was administered to Fischer 344 rats at doses of 0.005, 0.02, 0.05, and 0.1% in the diet (approximately 2.4, 9.5, 24.1 and 49.4 mg/kg/day for males and 3.0, 12.0, 30.1 and 62.8 mg/kg/day for females) for 24 months. No treatment-related tumors were noted in the rat carcinogenicity study in male or female rats up to the highest doses evaluated in this study of 24.1 mg/kg/day in male rats and 30.1 mg/kg/day in female rats. Rats in the highest dose group in this study were not evaluated due to high mortality.
Spinosad demonstrated no evidence of mutagenic or clastogenic potential based on the results of four in vitro genotoxicity tests (Ames assay, mouse lymphoma L5178Y assay, Chinese hamster ovary cell chromosome aberration assay, and rat hepatocyte unscheduled DNA synthesis assay) and one in vivo genotoxicity test (mouse bone marrow micronucleus assay).
Oral administration of spinosad (in diet) to rats, throughout mating, gestation, parturition and lactation, demonstrated no effects on growth, fertility or reproduction, at doses up to 10 mg/kg/day [see Use In Specific Populations (8.1)].
-
14 CLINICAL STUDIES
14.1 Head Lice Infestations
Two multicenter, randomized, investigator-blind, active-controlled trials were conducted in 1038 subjects 6 months of age and older with head lice infestation. A total of 552 subjects were treated with Spinosad Topical Suspension. For the evaluation of efficacy, the youngest subject from each household was considered to be the primary subject of the household, and other members in the household were enrolled in the study as secondary subjects and evaluated for all safety parameters.
In Study 1, 91 primary subjects were randomized to Spinosad Topical Suspension, and 89 primary subjects were randomized to permethrin 1%. In Study 2, 83 and 84 primary subjects were randomized to Spinosad Topical Suspension and permethrin 1%, respectively.
In both trials, all subjects who were treated on Day 0 returned for efficacy evaluation at Day 7. Subjects with live lice present at Day 7 received a second treatment. Subjects who were lice free on Day 7 were to return on Day 14 for evaluation. Subjects with live lice and who received a second treatment were to return on Days 14 and 21.
Efficacy was assessed as the proportion of primary subjects who were free of live lice 14 days after the final treatment. Table 3 contains the proportion of primary subjects who were free of live lice in each of the two trials.
Table 3. Proportion of Subjects Free of Live Lice 14 days After Last Treatment Study 1 Study 2 Spinosad
(N=91)Permethrin 1%
(N=89)Spinosad
(N=83)Permethrin 1%
(N=84)77 (84.6%) 40 (44.9%) 72 (86.7%) 36 (42.9%) 14.2 Scabies Infestations
Two multicenter, randomized, double-blind, vehicle-controlled trials (Trial 1 [NCT02485717] and Trial 2 [NCT02485704]) were conducted in subjects from 206 households in which at least one household member 4 years of age or older had an active scabies infestation. An active scabies infestation was defined as the presence of clinical signs and symptoms (evidence of burrows or presence of inflammatory/ noninflammatory lesions and pruritus) as well as microscopic evidence from a skin scraping or dermatoscopy to demonstrate the presence of mites, eggs, and/or scybala. All members of the household were treated with the same randomized treatment (Spinosad Topical Suspension or vehicle), whether or not the household member had an active scabies infestation. Subjects applied a single application of Spinosad Topical Suspension or vehicle on Day 1 and returned for evaluation on Day 28.
The two studies enrolled 533 adult and pediatric subjects 4 years of age and older. A total of 286 subjects (212 adults and 74 pediatrics) were treated with Spinosad Topical Suspension and 247 subjects (176 adults and 71 pediatrics) were treated with vehicle. For the evaluation of efficacy, the youngest subject from each household with an active scabies infestation was considered to be the primary subject of the household, and other members in the household were enrolled in the trial as secondary subjects and evaluated for all safety parameters. In Trial 1, 43 primary subjects were randomized to Spinosad Topical Suspension, and 43 primary subjects were randomized to vehicle. In Trial 2, 62 and 58 primary subjects were randomized to Spinosad Topical Suspension and vehicle, respectively.
Efficacy was assessed as the proportion of primary subjects who achieved complete cure 28 days after treatment. Complete cure is defined as a demonstration of clinical cure (all signs and symptoms have completely resolved, including burrows, inflammatory/non-inflammatory lesions and pruritus) and microscopic or dermatoscopic cure demonstrating the absence of mites, eggs, and/or scybala, and negative dermatoscopy for burrows. Table 4 contains the proportion of primary subjects who achieved complete cure in each of the two trials.
Table 4. Proportion of Primary Subjects with Scabies Infestation who Achieved Complete Cure 28 Days After Treatment Trial 1 Trial 2 Spinosad
N=43Vehicle
N=43Estimated Difference
95% CISpinosad
N=62Vehicle
N=58Estimated Difference
95% CICI= Confidence Interval 30 (69.8%) 20 (46.5%) 22.7%
(1.8%, 43.5%)52 (83.9%) 20 (34.5%) 49.7%
(36.0%, 63.5%) -
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Spinosad Topical Suspension, 0.9% w/w; each gram contains 9 mg of spinosad in a slightly opaque (white soft particles may be visible), light orange-colored, viscous liquid, supplied in 4 oz (120 mL) high density polyethylene (HDPE) bottles. NDC: 52246-570-04
-
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information)
Important Administration Instructions
Instruct the patient to:
- Shake bottle well immediately prior to application.
- Do not swallow.
- Avoid contact with eyes. If Spinosad Topical Suspension gets in or near the eyes, rinse thoroughly with water.
- Apply Spinosad Topical Suspension on pediatric patient only under direct supervision of an adult.
- If skin/scalp irritation occurs after use, contact your physician [see Adverse Reactions (6.1)] .
Head Lice Infestation Treatment
- Apply Spinosad Topical Suspension only on dry scalp and dry scalp hair.
- Repeat treatment only if live lice are seen seven (7) days after first treatment.
- Wash hands after applying Spinosad Topical Suspension.
Scabies Infestation Treatment
- Apply Spinosad Topical Suspension to completely cover the body from the neck to the toes (including the soles of the feet).
- For patients with balding scalp, also apply product to the scalp, hairline, temples, and forehead.
- Allow it to absorb in the skin and dry for 10 minutes before getting dressed.
- Leave on the skin for at least 6 hours before showering or bathing.
- Wash your hands after applying Spinosad Topical Suspension to someone else.
- For breastfeeding women, remove Spinosad Topical Suspension from the breast with soap and water before breastfeeding to avoid direct infant exposure to Spinosad Topical Suspension [see Use in Specific Populations (8.2)]
-
PATIENT PACKAGE INSERT
Patient Information for Head Lice Treatment
Spinosad Topical SuspensionImportant: For use on scalp hair and scalp only. Do not get Spinosad Topical Suspension in your eyes, mouth, or vagina. What is Spinosad Topical Suspension? Spinosad Topical Suspension is a prescription medicine used to treat head lice on the scalp and hair of adults and children 6 months of age and older.
It is not known if Spinosad Topical Suspension is safe and effective for children under 6 months of age.
See "How do I stop the spread of lice?" at the end of this leaflet for additional information on ways to stop the spread of lice.Before you use Spinosad Topical Suspension, tell your healthcare provider about all of your medical conditions, including if you or your child: - have any skin conditions or sensitivities
- are pregnant or plan to become pregnant. It is not known if Spinosad Topical Suspension can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby during treatment with Spinosad Topical Suspension.
How should I use Spinosad Topical Suspension? - See the detailed "Instructions for Use" at the end of this leaflet.
- Apply Spinosad Topical Suspension exactly as prescribed by your healthcare provider. Your healthcare provider will prescribe the treatment that is right for you. Do not change your treatment unless you talk to your healthcare provider.
- Apply Spinosad Topical Suspension when your hair is dry. Do not wet your hair before applying Spinosad Topical Suspension.
- It is important to apply enough Spinosad Topical Suspension to completely coat all of your hair and scalp. Leave Spinosad Topical Suspension on your hair and scalp for a full 10 minutes.
- If live lice are seen one week (7 days) after you applied Spinosad Topical Suspension, you will need to apply Spinosad Topical Suspension again.
- Because you need to completely cover all of the lice with Spinosad Topical Suspension, you may need help in applying Spinosad Topical Suspension to your scalp and hair.
- Children will need an adult to apply Spinosad Topical Suspension for them.
- Do not get Spinosad Topical Suspension into your eyes. If Spinosad Topical Suspension gets in your eye, rinse well with water right away.
- If you have any scalp irritation after you apply Spinosad Topical Suspension, call your healthcare provider right away.
- Wash your hands after you apply Spinosad Topical Suspension.
- Do not swallow Spinosad Topical Suspension. If swallowed, call Poison Control at 1-800-222-1222 or go to the nearest emergency room right away.
What are the possible side effects of Spinosad Topical Suspension? The most common side effects of Spinosad Topical Suspension include redness where Spinosad Topical Suspension is applied and redness to eyes. These are not all the possible side effects of Spinosad Topical Suspension. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store Spinosad Topical Suspension? - Store Spinosad Topical Suspension at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Spinosad Topical Suspension and all medicines out of the reach of children. General information about the safe and effective use of Spinosad Topical Suspension. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Spinosad Topical Suspension for a condition for which it was not prescribed. Do not give Spinosad Topical Suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Spinosad Topical Suspension that is written for health professionals. What are the ingredients in Spinosad Topical Suspension? Active ingredient: spinosad Inactive ingredients: Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth-20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster. -
Instructions for Use
Before you use Spinosad Topical Suspension, it is important that you read this Instructions for Use. Be sure that you read, understand, and follow this Instructions for Use so that you use Spinosad Topical Suspension the right way. Ask your healthcare provider or pharmacist if you have questions about the right way to use Spinosad Topical Suspension.
Important information:
- Your hair and scalp must be dry before applying Spinosad Topical Suspension.
- For very thick, medium length hair or long hair, an entire bottle (120mL) of Spinosad Topical Suspension may be needed to cover the scalp and hair. Less Spinosad Topical Suspension may be needed for shorter, thinner hair.
How to apply Spinosad Topical Suspension to your scalp and hair:
Step 1
- Shake Spinosad Topical Suspension bottle well right before use.
Step 2
- Cover your face and eyes with a towel and keep your eyes closed tightly.
- Apply Spinosad Topical Suspension directly to dry hair and scalp.
- Completely cover the scalp and hair closest to the scalp first, and then apply outwards towards the ends of the hair.
- It is important to apply enough Spinosad Topical Suspension to cover your entire scalp and hair so that all lice and eggs are exposed to Spinosad Topical Suspension.
Step 3

- Allow Spinosad Topical Suspension to stay on your scalp and hair for 10 minutes. Use a timer or clock and start timing after you have completely covered your hair and scalp with Spinosad Topical Suspension.
- Continue to keep eyes covered to prevent dripping into your eyes.
- After 10 minutes, completely rinse Spinosad Topical Suspension from your hair and scalp with warm water.
- You may use a fine-tooth comb to remove treated lice and nits from the hair and scalp, but combing is not required.
- Wash your hands after applying Spinosad Topical Suspension.
- It is okay to shampoo your hair any time after the treatment.
If you see live lice on your scalp or hair one week (7 days), after your first treatment, repeat the steps above.
How do I stop the spread of lice?
To help prevent the spread of lice from one person to another, here are some steps you can take:
- Avoid direct head-to-head contact with anyone known to have live, crawling lice.
- Do not share combs, brushes, hats, scarves, bandannas, ribbons, barrettes, hair bands, towels, helmets, or other hair-related personal items with anyone else, whether they have lice or not.
- Avoid sleepovers and slumber parties during lice outbreaks. Lice can live in bedding, pillows, and carpets that have recently been used by someone with lice.
- After finishing treatment with lice medicine, check everyone in your family for lice after one week. Be sure to talk to your healthcare provider about treatments for those who have lice.
- Machine-wash or dry-clean any bedding, towels and clothing used by anyone having lice. Machine-wash at high temperatures (150°F) and tumble in a hot dryer for 20 minutes.
- Wash personal items such as combs, brushes, and hair clips in hot water.
Distributed by: ParaPRO LLC, Brownsburg, IN 46112
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Revised: 01/2024 -
PATIENT PACKAGE INSERT
Patient Information for Scabies Treatment
Spinosad Topical SuspensionImportant: For use on skin only. Do not get Spinosad Topical Suspension in your eyes, mouth, or vagina. What is Spinosad Topical Suspension? Spinosad Topical Suspension is a prescription medicine used to treat scabies in adults and children 4 years of age and older. It is not known if Spinosad Topical Suspension is safe and effective in children under 4 years of age. See "How do I stop the spread of scabies?" at the end of this leaflet. Before you use Spinosad Topical Suspension, tell your healthcare provider about all of your medical conditions, including if you or your child: - have any skin conditions or sensitivities
- are pregnant or plan to become pregnant. It is not known if Spinosad Topical Suspension can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Wash the breast area with soap and water to remove Spinosad Topical Suspension before breastfeeding to avoid exposure to your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with Spinosad Topical Suspension.
How should I use Spinosad Topical Suspension? - See the detailed "Instructions for Use" at the end of this leaflet.
- Apply Spinosad Topical Suspension exactly as prescribed by your healthcare provider. Your healthcare provider will prescribe the treatment that is right for you. Do not change your treatment unless you talk to your healthcare provider.
- It is important to apply enough Spinosad Topical Suspension to completely cover your body from your neck to the soles of your feet. Make sure you apply to folds of skin, between fingers and toes, and under finger and toe nails.
- If you are balding, apply Spinosad Topical Suspension to the scalp, hairline, temples, and forehead.
- Wait a full 10 minutes to allow Spinosad Topical Suspension to soak into the skin and dry before getting dressed. Leave Spinosad Topical Suspension on the skin for at least 6 hours before showering or bathing.
- Because you need to completely cover your body from the neck down with Spinosad Topical Suspension, you may need help in applying Spinosad Topical Suspension to ensure full coverage.
- Children will need an adult to apply Spinosad Topical Suspension for them.
- Do not get Spinosad Topical Suspension into your eyes. If Spinosad Topical Suspension gets in your eye, rinse well with water right away.
- If you have any skin irritation after you apply Spinosad Topical Suspension, call your healthcare provider right away.
- Wash your hands after applying Spinosad Topical Suspension to someone else.
- Do not swallow Spinosad Topical Suspension. If swallowed, call Poison Control at 1-800-222-1222 or go to the nearest emergency room right away.
What are the possible side effects of Spinosad Topical Suspension? The most common side effects of Spinosad Topical Suspension include irritation (including pain and burning) at application sites and dry skin. These are not all the possible side effects of Spinosad Topical Suspension.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store Spinosad Topical Suspension? - Store Spinosad Topical Suspension at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Spinosad Topical Suspension and all medicines out of the reach of children. General information about the safe and effective use of Spinosad Topical Suspension. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Spinosad Topical Suspension for a condition for which it was not prescribed. Do not give Spinosad Topical Suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Spinosad Topical Suspension that is written for health professionals. What are the ingredients in Spinosad Topical Suspension? Active ingredient: spinosad Inactive ingredients: Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth-20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster. -
Instructions for Use
Before you use Spinosad Topical Suspension, it is important that you read this Instructions for Use. Be sure that you read, understand, and follow this Instructions for Use so that you use Spinosad Topical Suspension the right way. Ask your healthcare provider or pharmacist if you have questions about the right way to use Spinosad Topical Suspension.
How to apply Spinosad Topical Suspension to your body:
Step 1
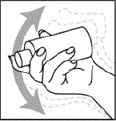
- Shake the Spinosad Topical Suspension bottle well right before use.
Step 2
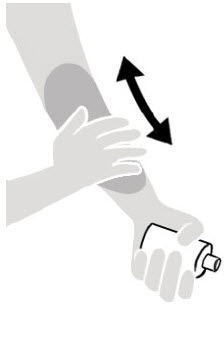
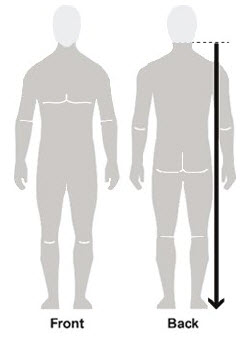
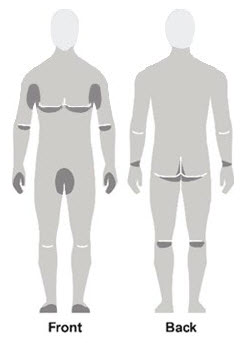
Figure A Figure B Figure C - Apply Spinosad Topical Suspension to the skin. (See Figure A)
- Apply enough Spinosad Topical Suspension to completely cover your body from your neck to the soles of your feet. (See Figure B). Make sure you apply to folds of skin, between fingers and toes, and under finger and toe nails. (See Figure C)
- If you are balding, you should also apply Spinosad Topical Suspension to the scalp, hairline, temples and forehead.
- You may need help applying Spinosad Topical Suspension to ensure full coverage.
- If you do not completely cover your body with Spinosad Topical Suspension, some scabies mites may escape treatment.
- Wash your hands after applying Spinosad Topical Suspension to someone else.
Step 3
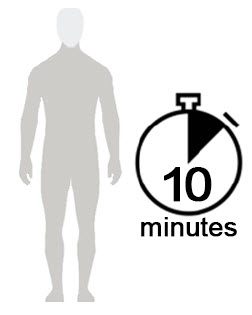
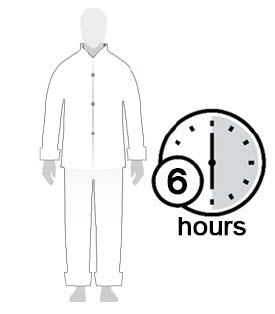
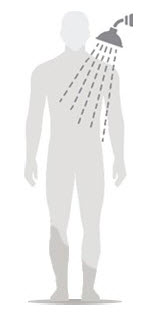
Figure D Figure E Figure F - Allow Spinosad Topical Suspension to soak into the skin and dry for 10 minutes. Use a timer or clock and start timing after you have completely covered your body with Spinosad Topical Suspension. (See Figure D)
- After 10 minutes, you can get dressed. (See Figure E)
- Wait at least 6 hours before showering or bathing. (See Figure F)
How do I stop the spread of scabies?
To help prevent the spread of scabies from one person to another, here are some steps you can take:
- Get household members checked by their healthcare provider.
- Avoid direct body-to-body contact with anyone known to have scabies, including sexual partners.
- Do not share clothing, towels, bedding or linens with anyone else. The scabies mite can live in bedding, pillows, and carpets that have recently been used by someone with scabies.
- Machine-wash or dry clean any bedding, clothing and towels that you have used anytime during the three days before treatment by washing in hot water and drying in a hot dryer, or by sealing in a plastic bag for at least 72 hours. Machine-wash at high temperatures (150°F) and tumble in a hot dryer for 20 minutes.
Distributed by: ParaPRO LLC, Brownsburg, IN 46112
SPN-PI-003
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Issued: 01/2024 -
PRINCIPAL DISPLAY PANEL - 120 mL Carton

NDC: 52246-570-04
Rx OnlySpinosad
Topical Suspension
0.9% w/wFor topical use only
120 mL
DIRECTIONS FOR USE
See package insert, including the patient information section, for full prescribing and dosing information.
SHAKE WELL BEFORE USE.
WARNINGS:
- Keep out of the reach of children.
- Spinosad Topical Suspension should be used on children under direct supervision of an adult.
- Do not swallow.
- Avoid contact with eyes. If Spinosad Topical Suspension gets into the eyes, immediately flush with water.
- If scalp irritation or infection occurs after use contact a physician.
Manufactured for: ParaPRO, LLC, Carmel, IN 46032
Distributed by: ParaPRO, LLC, Brownsburg, IN 46112
ParaPRO is a registered trademark of ParaPRO, LLC.
Spinosad Topical Suspension contains 9 mg spinosad per gram of compound consisting of Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth -20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
-
PRINCIPAL DISPLAY PANEL - 120 mL Bottle
NDC: 52246-570-04
Rx OnlySpinosad
Topical Suspension
0.9% w/wFor topical use only
120 mL
DIRECTIONS FOR USE
See package insert, including the patient information section, for full prescribing and dosing information.
SHAKE WELL BEFORE USE.
WARNINGS:
- Keep out of the reach of children.
- Spinosad Topical Suspension should be used on children under direct supervision of an adult.
- Do not swallow.
- Avoid contact with eyes. If Spinosad Topical Suspension gets into the eyes, immediately flush with water.
- If scalp irritation or infection occurs after use contact a physician.
Manufactured for: ParaPRO, LLC, Carmel, IN 46032
Distributed by: ParaPRO, LLC, Brownsburg, IN 46112
ParaPRO is a registered trademark of ParaPRO, LLC.
Spinosad Topical Suspension contains 9 mg spinosad per gram of compound consisting of Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth -20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
-
INGREDIENTS AND APPEARANCE
SPINOSAD
spinosad suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52246-570 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPINOSAD (UNII: XPA88EAP6V) (SPINOSAD - UNII:XPA88EAP6V) SPINOSAD 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYDROCHLORIC ACID (UNII: QTT17582CB) STEARALKONIUM CHLORIDE (UNII: 0OUO26BB88) WATER (UNII: 059QF0KO0R) 492 mg in 1 mL HEXYLENE GLYCOL (UNII: KEH0A3F75J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BENZYL ALCOHOL (UNII: LKG8494WBH) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange (PEARLESCENT LIGHT ORANGE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52246-570-04 1 in 1 CARTON 01/29/2024 1 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA022408 01/29/2024 Labeler - ParaPRO LLC (186639006) Establishment Name Address ID/FEI Business Operations Ferndale Laboratories, Inc. 005320536 manufacture(52246-570) , pack(52246-570) Establishment Name Address ID/FEI Business Operations Pharmaceutics International Inc. 878265586 manufacture(52246-570) , pack(52246-570)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.