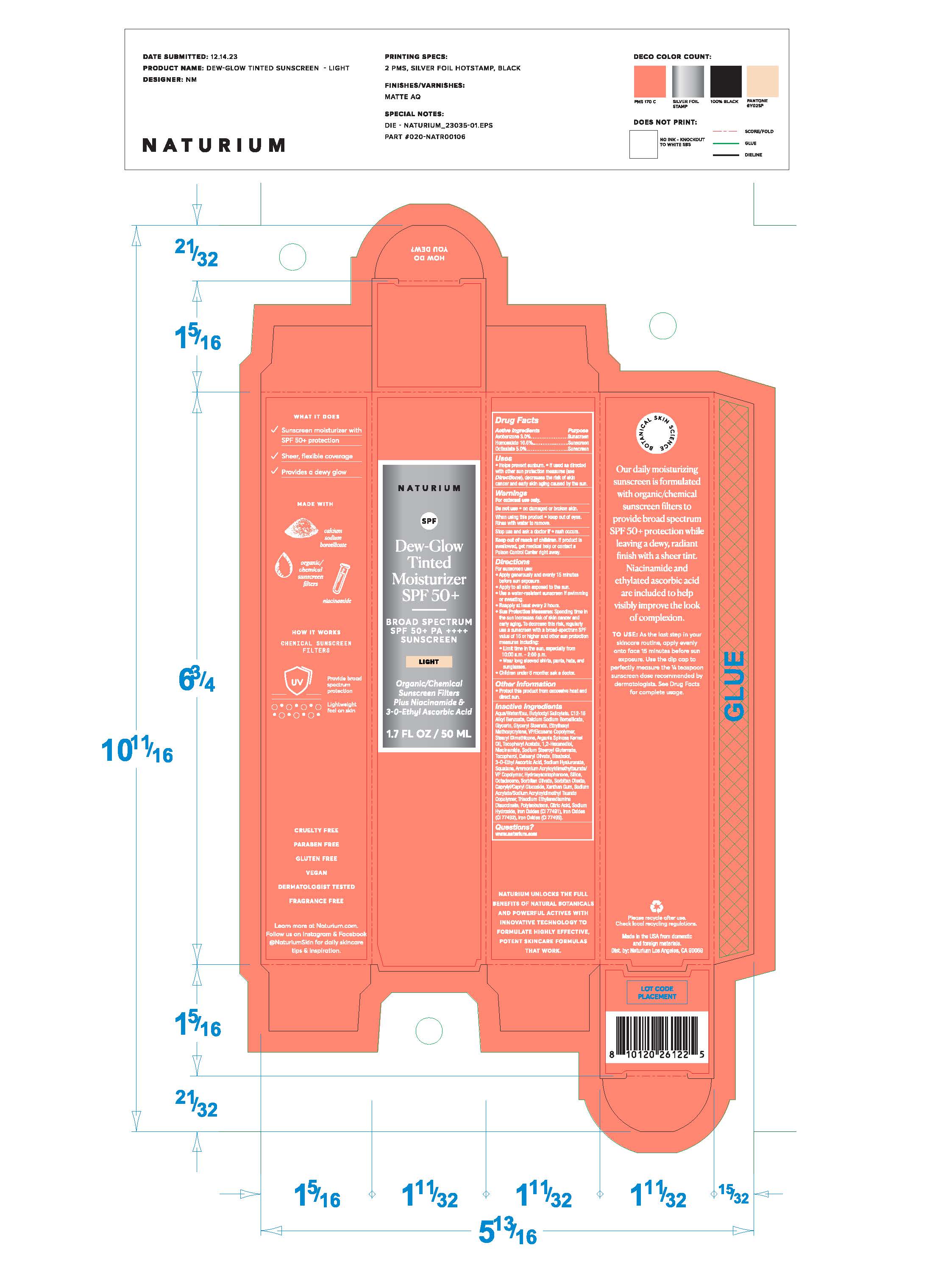
NATURIUM DEW GLOW TINTED MOISTURIZER SPF50- homosalate, octisalate, avobenzone lotion
Naturium Dew Glow Tinted Moisturizer SPF50 by
Drug Labeling and Warnings
Naturium Dew Glow Tinted Moisturizer SPF50 by is a Otc medication manufactured, distributed, or labeled by e.l.f. Cosmetics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- WARNINGS
-
Directions
For sunscreen use:
Apply generously and evenly 15 minutes before sun exposure.
Apply to all skin exposed to the sun.
Use a water-resistant sunscreen if swimming or sweating.
Reapply at least every 2 hours.
Sun Protection Measures: Spending time in the sun increases risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10:00 a.m. - 2:00 p.m.
Wear long sleeved shirts, pants, hats, and sunglasses.
Children under 6 months of age: Ask a doctor.
- Other Information
-
INACTIVE INGREDIENT
Aqua/Water/Eau, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Calcium Sodium Borosilicate, Glycerin, Glyceryl Stearate, Ethylhexyl Methoxycrylene, VP/Eicosene Copolymer, Stearyl Dimethicone, Argania Spinosa Kernel Oil, Tocopheryl Acetate, 1,2-Hexandiol, Niacinamide, Sodium Stearoyl Glutamate, Tocopherol, Cetearyl Olivate, Bisabolol, 3-O-Ethyl Ascorbic Acid, Sodium Hyaluronate, Squalane, Ammonium Acryloyldimethyltaurate/VP Copolymer, Hydroxyacetophnone, Silica, Octadecene, Sorbitan Olivate, Sorbitan Oleate, Caprylyl/Capryl Glucoside, Xanthan Gum, Sodium Acrylate/Sodium Acryloyldimethyltaurate/VP Copolymer, Hydroxyacetophenone, Silica, Octadecene, Sorbitan Olivate, Sorbitan Oleate, Caprylyl/Capryl Glucoside, Xanthan Gum, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Trisodium Ethylenediamine Disuccinate, Polyisobutene, Citric Acid, Sodium Hydroxide
May Contain: Iron Oxide (CI 77491), Iron Oxide (CI 77492), Iron Oxide (CI 77499)
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATURIUM DEW GLOW TINTED MOISTURIZER SPF50
homosalate, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76354-131 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 50 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 50 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 50 mL Inactive Ingredients Ingredient Name Strength 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) NIACINAMIDE (UNII: 25X51I8RD4) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TOCOPHEROL (UNII: R0ZB2556P8) OCTADECENE (UNII: H5ZUQ6V4AK) XANTHAN GUM (UNII: TTV12P4NEE) POLYISOBUTYLENE (400000 MW) (UNII: X9N69O5R5X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CETEARYL OLIVATE (UNII: 58B69Q84JO) BROWN IRON OXIDE (UNII: 1N032N7MFO) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONIC ACID (UNII: S270N0TRQY) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) BOROSILICATE GLASS (UNII: BOJ6T9AR90) ARGAN OIL (UNII: 4V59G5UW9X) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SQUALANE (UNII: GW89575KF9) SORBITAN OLIVATE (UNII: MDL271E3GR) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76354-131-01 1 in 1 CARTON 12/27/2023 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/27/2023 Labeler - e.l.f. Cosmetics, Inc. (093902816)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.