AVALIDE- irbesartan and hydrochlorothiazide tablet, film coated
Avalide by
Drug Labeling and Warnings
Avalide by is a Prescription medication manufactured, distributed, or labeled by sanofi-aventis U.S. LLC, Sanofi Chimie, EUROAPI Hungary Ltd., Eurofins Lancaster Laboratories, Inc, CAMBREX PROFARMACO MILANO S.R.L., Sanofi Winthrop Industrie. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AVALIDE safely and effectively. See full prescribing information for AVALIDE.
AVALIDE® (irbesartan and hydrochlorothiazide) tablets, for oral use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
AVALIDE is a combination of irbesartan, an angiotensin II receptor antagonist, and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension:
DOSAGE AND ADMINISTRATION
General Considerations
- Maximum effects within 2 to 4 weeks after dose change. (2.1)
- Renal impairment: Not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min). (2.1, 5.8)
Hypertension
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse events (≥5% on AVALIDE and more often than on placebo) are dizziness, fatigue, and musculoskeletal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- NSAIDs and selective COX-2 inhibitors: Can reduce diuretic, natriuretic of diuretic, may lead to increased risk of renal impairment and reduced antihypertensive effect. Monitor renal function periodically. (7)
- Dual blockade of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia. (7)
- Antidiabetic drugs: Dosage adjustment of antidiabetic may be required. (7)
- Cholestyramine and colestipol: Reduced absorption of thiazides. (7)
- Lithium: Increases in serum lithium concentrations and lithium toxicity. (7)
- Carbamazepine: Increased risk of hyponatremia. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
2.2 Add-On Therapy
2.3 Replacement Therapy
2.4 Initial Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Hypotension in Volume or Salt-Depleted Patients
5.3 Hypersensitivity Reaction
5.4 Systemic Lupus Erythematosus
5.5 Electrolyte and Metabolic Imbalances
5.6 Hepatic Impairment
5.7 Impaired Renal Function
5.8 Secondary Acute Angle-Closure Glaucoma and/or Acute Myopia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Laboratory Abnormalities
7 DRUG INTERACTIONS
7.1 Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
7.2 Dual Blockade of the Renin-Angiotensin System (RAS)
7.3 Agents Increasing Serum Potassium
7.4 Antidiabetic Drugs (oral agents and insulin)
7.5 Cholestyramine and Colestipol Resins
7.6 Lithium
7.7 Carbamazepine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Irbesartan Monotherapy
14.2 Irbesartan-Hydrochlorothiazide
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue AVALIDE as soon as possible. [See Warnings and Precautions (5.1).]
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. [See Warnings and Precautions (5.1).]
-
1 INDICATIONS AND USAGE
AVALIDE® (irbesartan-hydrochlorothiazide) tablets are indicated for the treatment of hypertension.
AVALIDE may be used in patients whose blood pressure is not adequately controlled on monotherapy.
AVALIDE may also be used as initial therapy in patients who are likely to need multiple drugs to achieve their blood pressure goals.
The choice of AVALIDE as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Patients with stage 2 (moderate or severe) hypertension are at relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and may be shaped by considerations such as the baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared with monotherapy.
Data from Studies V and VI [see Clinical Studies (14.2)] provide estimates of the probability of reaching a blood pressure goal with AVALIDE compared to irbesartan or HCTZ monotherapy. The relationship between baseline blood pressure and achievement of a SeSBP <140 or <130 mmHg or SeDBP <90 or <80 mmHg in patients treated with AVALIDE compared to patients treated with irbesartan or HCTZ monotherapy are shown in Figures 1a through 2b.
- * For all probability curves, patients without blood pressure measurements at Week 7 (Study VI) and Week 8 (Study V) were counted as not reaching goal (intent-to-treat analysis).
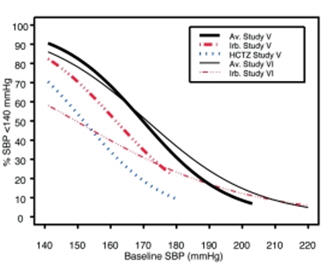
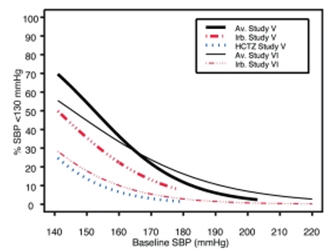
Figure 1a: Probability of Achieving SBP <140 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* Figure 1b: Probability of Achieving SBP <130 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* 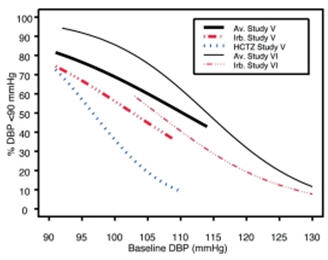
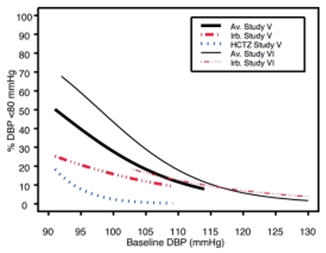
Figure 2a: Probability of Achieving DBP <90 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* Figure 2b: Probability of Achieving DBP <80 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* The above graphs provide a rough approximation of the likelihood of reaching a targeted blood pressure goal (e.g., Week 8 sitting systolic blood pressure ≤140 mmHg) for the treatment groups. The curve of each treatment group in each study was estimated by logistic regression modeling from all available data of that treatment group. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures.
For example, a patient with a blood pressure of 180/105 mmHg has about a 25% likelihood of achieving a goal of <140 mmHg (systolic) and 50% likelihood of achieving <90 mmHg (diastolic) on irbesartan alone (and lower still likelihoods on HCTZ alone).
The likelihood of achieving these goals on AVALIDE rises to about 40% (systolic) or 70% (diastolic).
-
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
The side effects of irbesartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. [See Adverse Reactions (6).]
Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.
AVALIDE may be administered with or without food.
AVALIDE may be administered with other antihypertensive agents.
2.2 Add-On Therapy
In patients not controlled on monotherapy with irbesartan or hydrochlorothiazide, the recommended doses of AVALIDE, in order of increasing mean effect, are (irbesartan-hydrochlorothiazide) 150/12.5 mg, 300/12.5 mg, and 300/25 mg. The largest incremental effect will likely be in the transition from monotherapy to 150/12.5 mg. [See Clinical Studies (14.2).]
2.4 Initial Therapy
The usual starting dose is AVALIDE 150/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of 300/25 mg once daily as needed to control blood pressure [See Clinical Studies (14.2)]. AVALIDE is not recommended as initial therapy in patients with intravascular volume depletion [See Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- AVALIDE is contraindicated in patients who are hypersensitive to any component of this product.
- Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
- Do not coadminister aliskiren with AVALIDE in patients with diabetes [See Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue AVALIDE as soon as possible [See Use in Specific Populations (8.1)].
Thiazides cross the placenta, and use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
5.2 Hypotension in Volume or Salt-Depleted Patients
Excessive reduction of blood pressure was rarely seen in patients with uncomplicated hypertension treated with irbesartan alone (<0.1%) or with irbesartan-hydrochlorothiazide (approximately 1%). Initiation of antihypertensive therapy may cause symptomatic hypotension in patients with intravascular volume or sodium-depletion, e.g., in patients treated vigorously with diuretics or in patients on dialysis. Such volume depletion should be corrected prior to administration of antihypertensive therapy.
If hypotension occurs, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.5 Electrolyte and Metabolic Imbalances
Irbesartan-Hydrochlorothiazide
In double-blind clinical trials of various doses of irbesartan and hydrochlorothiazide, the incidence of hypertensive patients who developed hypokalemia (serum potassium <3.5 mEq/L) was 7.5% versus 6.0% for placebo; the incidence of hyperkalemia (serum potassium >5.7 mEq/L) was <1.0% versus 1.7% for placebo. No patient discontinued due to increases or decreases in serum potassium. On average, the combination of irbesartan and hydrochlorothiazide had no effect on serum potassium. Higher doses of irbesartan ameliorated the hypokalemic response to hydrochlorothiazide.
Coadministration of AVALIDE with potassium sparing diuretics, potassium supplements, potassium-containing salt substitutes or other drugs that raise serum potassium levels may result in hyperkalemia, sometimes severe. Monitor serum potassium in such patients.
Hydrochlorothiazide
Hydrochlorothiazide can cause hypokalemia and hyponatremia. Hypomagnesemia can result in hypokalemia which appears difficult to treat despite potassium repletion. Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Monitor serum electrolytes periodically.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for parathyroid function.
5.7 Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals [See Drug Interactions (7)]. In patients whose renal function may depend on the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors has been associated with oliguria and/or progressive azotemia and (rarely) with acute renal failure and/or death. Irbesartan would be expected to behave similarly. In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or BUN have been reported. There has been no known use of irbesartan in patients with unilateral or bilateral renal artery stenosis, but a similar effect should be anticipated.
Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
5.8 Secondary Acute Angle-Closure Glaucoma and/or Acute Myopia
Hydrochlorothiazide
Sulfonamide or sulfonamide derivative drugs, such as hydrochlorothiazide, can cause an idiosyncratic reaction, resulting in transient myopia and/or acute angle-closure glaucoma. Cases of acute angle-closure glaucoma have been reported with hydrochlorothiazide. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue drug intake as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Irbesartan-Hydrochlorothiazide
AVALIDE tablets have been evaluated for safety in 1694 patients treated for essential hypertension in 6 clinical trials. In Studies I through IV with AVALIDE, no adverse events peculiar to this combination drug product have been observed. Adverse events have been limited to those that were reported previously with irbesartan or hydrochlorothiazide (HCTZ). The overall incidence of adverse events was similar with the combination and placebo. In general, treatment with AVALIDE was well tolerated. For the most part, adverse events have been mild and transient in nature and have not required discontinuation of therapy. In controlled clinical trials, discontinuation of AVALIDE therapy due to clinical adverse events was required in only 3.6%. This incidence was significantly less (p=0.023) than the 6.8% of patients treated with placebo who discontinued therapy.
In these double-blind controlled clinical trials, the following adverse events reported with AVALIDE occurred in ≥1% of patients, and more often on the irbesartan-hydrochlorothiazide combination than on placebo, regardless of drug relationship:
Irbesartan/HCTZ
(n=898)
(%)Placebo
(n=236)
(%)Irbesartan
(n=400)
(%)HCTZ
(n=380)
(%)Body as a Whole Chest Pain 2 1 2 2 Fatigue 6 3 4 3 Influenza 3 1 2 2 Cardiovascular Edema 3 3 2 2 Tachycardia 1 0 1 1 Gastrointestinal Abdominal Pain 2 1 2 2 Dyspepsia/heartburn 2 1 0 2 Nausea/vomiting 3 0 2 2 Immunology Allergy 1 0 1 1 Musculoskeletal Musculoskeletal Pain 6 5 6 10 Nervous System Dizziness 8 4 6 5 Dizziness Orthostatic 1 0 1 1 Renal/Genitourinary Abnormality Urination 2 1 1 2 The following adverse events were also reported at a rate of 1% or greater, but were as, or more, common in the placebo group: headache, sinus abnormality, cough, URI, pharyngitis, diarrhea, rhinitis, urinary tract infection, rash, anxiety/nervousness, and muscle cramp.
Adverse events occurred at about the same rates in men and women, older and younger patients, and black and non-black patients.
Adverse events in Studies V and VI were similar to those described above in Studies I through IV.
Irbesartan
Other adverse events that have been reported with irbesartan, without regard to causality, are listed below:
Body as a Whole: fever, chills, orthostatic effects, facial edema, upper extremity edema
Cardiovascular: flushing, hypertension, cardiac murmur, myocardial infarction, angina pectoris, hypotension, syncope, arrhythmic/conduction disorder, cardiorespiratory arrest, heart failure, hypertensive crisis
Dermatologic: pruritus, dermatitis, ecchymosis, erythema face, urticaria
Endocrine/Metabolic/Electrolyte Imbalances: sexual dysfunction, libido change, gout
Gastrointestinal: diarrhea, constipation, gastroenteritis, flatulence, abdominal distention
Musculoskeletal/Connective Tissue: musculoskeletal trauma, extremity swelling, muscle cramp, arthritis, muscle ache, musculoskeletal chest pain, joint stiffness, bursitis, muscle weakness
Nervous System: anxiety/nervousness, sleep disturbance, numbness, somnolence, vertigo, emotional disturbance, depression, paresthesia, tremor, transient ischemic attack, cerebrovascular accident
Renal/Genitourinary: prostate disorder
Respiratory: cough, upper respiratory infection, epistaxis, tracheobronchitis, congestion, pulmonary congestion, dyspnea, wheezing
Special Senses: vision disturbance, hearing abnormality, ear infection, ear pain, conjunctivitis
Hydrochlorothiazide
Other adverse events that have been reported with hydrochlorothiazide, without regard to causality, are listed below:
Body as a Whole: weakness
Digestive: pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation
Hematologic: aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity: purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions
Metabolic: hyperglycemia, glycosuria, hyperuricemia
Musculoskeletal: muscle spasm
Nervous System/Psychiatric: restlessness
Renal: renal failure, renal dysfunction, interstitial nephritis
Skin: erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis
Special Senses: transient blurred vision, xanthopsia
Initial Therapy
In the moderate hypertension Study V (mean SeDBP between 90 and 110 mmHg), the types and incidences of adverse events reported for patients treated with AVALIDE were similar to the adverse event profile in patients on initial irbesartan or HCTZ monotherapy. There were no reported events of syncope in the AVALIDE treatment group and there was one reported event in the HCTZ treatment group. The incidences of pre-specified adverse events on AVALIDE, irbesartan, and HCTZ, respectively, were: 0.9%, 0%, and 0% for hypotension; 3.0%, 3.8%, and 1.0% for dizziness; 5.5%, 3.8%, and 4.8% for headache; 1.2%, 0%, and 1.0% for hyperkalemia; and 0.9%, 0%, and 0% for hypokalemia. The rates of discontinuation due to adverse events on AVALIDE, irbesartan alone, and HCTZ alone were 6.7%, 3.8%, and 4.8%.
In the severe hypertension (SeDBP ≥110 mmHg) Study VI, the overall pattern of adverse events reported through 7 weeks of follow-up was similar in patients treated with AVALIDE as initial therapy and in patients treated with irbesartan as initial therapy. The incidences of the pre-specified adverse events on AVALIDE and irbesartan, respectively, were: 0% and 0% for syncope; 0.6% and 0% for hypotension; 3.6% and 4.0% for dizziness; 4.3% and 6.6% for headache; 0.2% and 0% for hyperkalemia; and 0.6% and 0.4% for hypokalemia. The rates of discontinuation due to adverse events were 2.1% and 2.2%. [See Clinical Studies (14.2).]
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AVALIDE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to AVALIDE.
The following have been very rarely reported with irbesartan and hydrochlorothiazide monotherapies: urticaria, jaundice, hepatitis, thrombocytopenia, and impaired renal function including renal failure.
The following have been reported with irbesartan monotherapy: tinnitus, hyperkalemia, angioedema (involving swelling of the face, lips, pharynx, and/or tongue), anaphylactic reaction including anaphylactic shock, and increased CPK.
The following have been reported with hydrochlorothiazide monotherapy: secondary acute angle-closure glaucoma and/or acute myopia.
6.3 Laboratory Abnormalities
In controlled clinical trials, clinically important changes in standard laboratory parameters were rarely associated with administration of AVALIDE.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen (BUN) or serum creatinine were observed in 2.3% and 1.1%, respectively, of patients with essential hypertension treated with AVALIDE alone. No patient discontinued taking AVALIDE due to increased BUN. One patient discontinued taking AVALIDE due to a minor increase in serum creatinine.
Liver Function Tests: Occasional elevations of liver enzymes and/or serum bilirubin have occurred. In patients with essential hypertension treated with AVALIDE alone, one patient was discontinued due to elevated liver enzymes.
Serum Electrolytes: [See Warnings and Precautions (5.2, 5.6).]
-
7 DRUG INTERACTIONS
7.1 Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Irbesartan
In patients who are elderly, volume depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including irbesartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Therefore, monitor renal function and blood pressure periodically in patients receiving irbesartan and NSAID therapy.
Hydrochlorothiazide
Administration of a non-steroidal anti-inflammatory agent, including a selective COX-2 inhibitor can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing, and thiazide diuretics. Therefore, when AVALIDE (irbesartan-hydrochlorothiazide) tablets and non-steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
7.2 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin-receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function, and electrolytes in patients on AVALIDE and other agents that affect the RAS.
In most patients no benefit has been associated with using two RAS inhibitors concomitantly. In general, avoid combined use of RAS inhibitors.
Do not coadminister aliskiren with AVALIDE in patients with diabetes. Avoid use of aliskiren with AVALIDE in patients with renal impairment (GFR <60 mL/min).
7.3 Agents Increasing Serum Potassium
Coadministration of AVALIDE with other drugs that raise serum potassium levels may result in hyperkalemia, sometimes severe. Monitor serum potassium in such patients.
7.4 Antidiabetic Drugs (oral agents and insulin)
Dosage adjustment of the antidiabetic drug may be required when coadministered with hydrochlorothiazide.
7.5 Cholestyramine and Colestipol Resins
Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Stagger the dosage of hydrochlorothiazide and the resin such that AVALIDE is administered at least 4 hours before or 4 to 6 hours after the administration of the resin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue AVALIDE as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue AVALIDE, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to AVALIDE for hypotension, oliguria, and hyperkalemia [See Use in Specific Populations (8.4)].
Irbesartan crosses the placenta in rats and rabbits. In pregnant rats given irbesartan at doses greater than the maximum recommended human dose (MRHD), fetuses showed increased incidences of renal pelvic cavitation, hydroureter and/or absence of renal papilla. Subcutaneous edema also occurred in fetuses at doses about 4 times the MRHD (based on body surface area). These anomalies occurred when pregnant rats received irbesartan through Day 20 of gestation but not when drug was stopped on gestation Day 15. The observed effects are believed to be late gestational effects of the drug. Pregnant rabbits given oral doses of irbesartan equivalent to 1.5 times the MRHD experienced a high rate of maternal mortality and abortion. Surviving females had a slight increase in early resorptions and a corresponding decrease in live fetuses [see Nonclinical Toxicology (13.2)].
Radioactivity was present in the rat and rabbit fetus during late gestation and in rat milk following oral doses of radiolabeled irbesartan.
When pregnant mice and rats were given hydrochlorothiazide at doses up to 3000 and 1000 mg/kg/day, respectively (about 600 and 400 times the MRHD) during their respective periods of major organogenesis, there was no evidence of fetal harm.
A development toxicity study was performed in rats with doses of 50/50 mg/kg/day and 150/150 mg/kg/day irbesartan-hydrochlorothiazide. Although the high dose combination appeared to be more toxic to the dams than either drug alone, there did not appear to be an increase in toxicity to the developing embryos.
8.3 Nursing Mothers
It is not known whether irbesartan is excreted in human milk, but irbesartan or some metabolite of irbesartan is secreted at low concentration in the milk of lactating rats.
Thiazides appear in human milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Neonates with a History of in utero Exposure to AVALIDE
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Of 1694 patients receiving AVALIDE in controlled clinical studies of hypertension, 264 (15.6%) were 65 years and over, while 45 (2.7%) were 75 years and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. [See Clinical Pharmacology (12.3) and Clinical Studies (14).]
-
10 OVERDOSAGE
Irbesartan
No data are available in regard to overdosage in humans. However, daily doses of 900 mg for 8 weeks were well tolerated. The most likely manifestations of overdosage are expected to be hypotension and tachycardia; bradycardia might also occur from overdose. Irbesartan is not removed by hemodialysis.
To obtain up-to-date information about the treatment of overdosage, a good resource is a certified regional Poison Control Center. Telephone numbers of certified Poison Control Centers are listed in the Physicians' Desk Reference (PDR). In managing overdose, consider the possibilities of multiple-drug interactions, drug-drug interactions, and unusual drug kinetics in the patient.
Laboratory determinations of serum levels of irbesartan are not widely available, and such determinations have, in any event, no established role in the management of irbesartan overdose.
Acute oral toxicity studies with irbesartan in mice and rats indicated acute lethal doses were in excess of 2000 mg/kg, about 25-fold and 50-fold the MRHD (300 mg) on a mg/m2 basis, respectively.
Hydrochlorothiazide
The most common signs and symptoms of overdose observed in humans are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats.
-
11 DESCRIPTION
AVALIDE (irbesartan-hydrochlorothiazide) tablets are a combination of an angiotensin II receptor antagonist (AT1 subtype), irbesartan, and a thiazide diuretic, hydrochlorothiazide (HCTZ).
Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and its structural formula is:
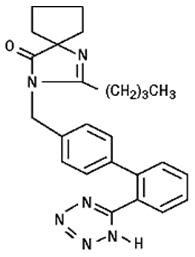
Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
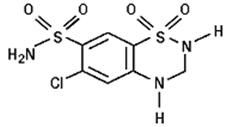
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium hydroxide solution.
AVALIDE is available for oral administration in film-coated tablets containing either 150 mg or 300 mg of irbesartan combined with 12.5 mg of hydrochlorothiazide. All dosage strengths contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hypromellose, magnesium stearate, silicon dioxide, ferric oxide red, ferric oxide yellow, polyethylene glycol, titanium dioxide, and carnauba wax.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Irbesartan
Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the RAS and also stimulates aldosterone synthesis and secretion by adrenal cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. Irbesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively binding to the AT1 angiotensin II receptor. There is also an AT2 receptor in many tissues, but it is not involved in cardiovascular homeostasis.
Irbesartan is a specific competitive antagonist of AT1 receptors with a much greater affinity (more than 8500-fold) for the AT1 receptor than for the AT2 receptor, and no agonist activity.
Blockade of the AT1 receptor removes the negative feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II do not overcome the effects of irbesartan on blood pressure.
Irbesartan does not inhibit ACE or renin or affect other hormone receptors or ion channels known to be involved in the cardiovascular regulation of blood pressure and sodium homeostasis. Because irbesartan does not inhibit ACE, it does not affect the response to bradykinin; whether this has clinical relevance is not known.
Hydrochlorothiazide
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so coadministration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is not fully understood.
12.2 Pharmacodynamics
Irbesartan
In healthy subjects, single oral irbesartan doses of up to 300 mg produced dose-dependent inhibition of the pressor effect of angiotensin II infusions. Inhibition was complete (100%) 4 hours following oral doses of 150 mg or 300 mg and partial inhibition was sustained for 24 hours (60% and 40% at 300 mg and 150 mg, respectively).
In hypertensive patients, angiotensin II receptor inhibition following chronic administration of irbesartan causes a 1.5-fold to 2-fold rise in angiotensin II plasma concentration and a 2-fold to 3-fold increase in plasma renin levels. Aldosterone plasma concentrations generally decline following irbesartan administration, but serum potassium levels are not significantly affected at recommended doses.
In hypertensive patients, chronic oral doses of irbesartan (up to 300 mg) had no effect on glomerular filtration rate, renal plasma flow or filtration fraction. In multiple dose studies in hypertensive patients, there were no clinically important effects on fasting triglycerides, total cholesterol, HDL-cholesterol, or fasting glucose concentrations. There was no effect on serum uric acid during chronic oral administration and no uricosuric effect.
Hydrochlorothiazide
After oral administration of hydrochlorothiazide, diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
12.3 Pharmacokinetics
Irbesartan
Irbesartan is an orally active agent that does not require biotransformation into an active form. The oral absorption of irbesartan is rapid and complete with an average absolute bioavailability of 60% to 80%. Following oral administration of irbesartan, peak plasma concentrations of irbesartan are attained at 1.5 to 2 hours after dosing. Food does not affect the bioavailability of irbesartan.
Irbesartan exhibits linear pharmacokinetics over the therapeutic dose range.
The terminal elimination half-life of irbesartan averaged 11 to 15 hours. Steady-state concentrations are achieved within 3 days. Limited accumulation of irbesartan (<20%) is observed in plasma upon repeated once-daily dosing.
Hydrochlorothiazide
When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours.
Metabolism and Elimination
Irbesartan
Irbesartan is metabolized via glucuronide conjugation and oxidation. Following oral or intravenous administration of 14C-labeled irbesartan, more than 80% of the circulating plasma radioactivity is attributable to unchanged irbesartan. The primary circulating metabolite is the inactive irbesartan glucuronide conjugate (approximately 6%). The remaining oxidative metabolites do not add appreciably to irbesartan's pharmacologic activity.
Irbesartan and its metabolites are excreted by both biliary and renal routes. Following either oral or intravenous administration of 14C-labeled irbesartan, about 20% of radioactivity is recovered in the urine and the remainder in the feces, as irbesartan or irbesartan glucuronide.
In vitro studies of irbesartan oxidation by cytochrome P450 isoenzymes indicated irbesartan was oxidized primarily by 2C9; metabolism by 3A4 was negligible. Irbesartan was neither metabolized by, nor did it substantially induce or inhibit, isoenzymes commonly associated with drug metabolism (1A1, 1A2, 2A6, 2B6, 2D6, 2E1). There was no induction or inhibition of 3A4.
Distribution
Irbesartan
Irbesartan is 90% bound to serum proteins (primarily albumin and α1-acid glycoprotein) with negligible binding to cellular components of blood. The average volume of distribution is 53 to 93 liters. Total plasma and renal clearances are in the range of 157 to 176 mL/min and 3.0 to 3.5 mL/min, respectively. With repetitive dosing, irbesartan accumulates to no clinically relevant extent.
Studies in animals indicate that radiolabeled irbesartan weakly crosses the blood-brain barrier and placenta. Irbesartan is excreted in the milk of lactating rats.
Specific Populations
Pediatric
Irbesartan-hydrochlorothiazide pharmacokinetics have not been investigated in patients <18 years of age.
Gender
No gender-related differences in pharmacokinetics were observed in healthy elderly (age 65 to 80 years) or in healthy young (age 18 to 40 years) subjects. In studies of hypertensive patients, there was no gender difference in half-life or accumulation, but somewhat higher plasma concentrations of irbesartan were observed in females (11% to 44%). No gender-related dosage adjustment is necessary.
Geriatric
In elderly subjects (age 65 to 80 years), irbesartan elimination half-life was not significantly altered, but AUC and Cmax values were about 20% to 50% greater than those of young subjects (age 18 to 40 years). No dosage adjustment is necessary in the elderly.
Race
In healthy black subjects, irbesartan AUC values were approximately 25% greater than whites; there were no differences in Cmax values.
Renal insufficiency
The pharmacokinetics of irbesartan were not altered in patients with renal impairment or in patients on hemodialysis. Irbesartan is not removed by hemodialysis. No dosage adjustment is necessary in patients with mild to severe renal impairment unless a patient with renal impairment is also volume depleted. [See Warnings and Precautions (5.2).]
Drug-Drug Interactions
No significant drug-drug pharmacokinetic (or pharmacodynamic) interactions have been found in interaction studies with hydrochlorothiazide, digoxin, warfarin, and nifedipine.
In vitro studies show significant inhibition of the formation of oxidized irbesartan metabolites with the known cytochrome CYP2C9 substrates/inhibitors sulphenazole, tolbutamide, and nifedipine. However, in clinical studies the consequences of concomitant irbesartan on the pharmacodynamics of warfarin were negligible. Concomitant nifedipine or hydrochlorothiazide had no effect on irbesartan pharmacokinetics. Based on in vitro data, no interaction would be expected with drugs whose metabolism is dependent upon cytochrome P450 isoenzymes 1A1, 1A2, 2A6, 2B6, 2D6, 2E1, or 3A4.
In separate studies of patients receiving maintenance doses of warfarin, hydrochlorothiazide, or digoxin, irbesartan administration for 7 days had no effect on the pharmacodynamics of warfarin (prothrombin time) or the pharmacokinetics of digoxin. The pharmacokinetics of irbesartan were not affected by coadministration of nifedipine or hydrochlorothiazide.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Irbesartan-Hydrochlorothiazide
No carcinogenicity studies have been conducted with the irbesartan-hydrochlorothiazide combination.
Irbesartan-hydrochlorothiazide was not mutagenic in standard in vitro tests (Ames microbial test and Chinese hamster mammalian-cell forward gene-mutation assay). Irbesartan-hydrochlorothiazide was negative in tests for induction of chromosomal aberrations (in vitro–human lymphocyte assay; in vivo–mouse micronucleus study).
The combination of irbesartan and hydrochlorothiazide has not been evaluated in definitive studies of fertility.
Irbesartan
No evidence of carcinogenicity was observed when irbesartan was administered at doses of up to 500/1000 mg/kg/day (males/females, respectively) in rats and 1000 mg/kg/day in mice for up to 2 years. For male and female rats, 500 mg/kg/day provided an average systemic exposure to irbesartan (AUC0–24 hours, bound plus unbound) about 3 and 11 times, respectively, the average systemic exposure in humans receiving the maximum recommended dose (MRD) of 300 mg irbesartan/day, whereas 1000 mg/kg/day (administered to females only) provided an average systemic exposure about 21 times that reported for humans at the MRD. For male and female mice, 1000 mg/kg/day provided an exposure to irbesartan about 3 and 5 times, respectively, the human exposure at 300 mg/day.
Irbesartan was not mutagenic in a battery of in vitro tests (Ames microbial test, rat hepatocyte DNA repair test, V79 mammalian-cell forward gene-mutation assay). Irbesartan was negative in several tests for induction of chromosomal aberrations (in vitro–human lymphocyte assay; in vivo–mouse micronucleus study).
Irbesartan had no adverse effects on fertility or mating of male or female rats at oral doses ≤650 mg/kg/day, the highest dose providing a systemic exposure to irbesartan (AUC0–24 hours, bound plus unbound) about 5 times that found in humans receiving the MRD of 300 mg/day.
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 µg/mL, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to mating and throughout gestation.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
When pregnant rats were treated with irbesartan from Day 0 to Day 20 of gestation (oral doses of 50, 180, and 650 mg/kg/day), increased incidences of renal pelvic cavitation, hydroureter, and/or absence of renal papilla were observed in fetuses at doses ≥50 mg/kg/day (approximately equivalent to the MRHD, 300 mg/day, on a body surface area basis). Subcutaneous edema was observed in fetuses at doses ≥180 mg/kg/day (about 4 times the MRHD on a body surface area basis). As these anomalies were not observed in rats in which irbesartan exposure (oral doses of 50, 150, and 450 mg/kg/day) was limited to gestation days 6 to 15, they appear to reflect late gestational effects of the drug. In pregnant rabbits, oral doses of 30 mg irbesartan/kg/day were associated with maternal mortality and abortion. Surviving females receiving this dose (about 1.5 times the MRHD on a body surface area basis) had a slight increase in early resorptions and a corresponding decrease in live fetuses. Irbesartan was found to cross the placental barrier in rats and rabbits.
-
14 CLINICAL STUDIES
14.1 Irbesartan Monotherapy
The antihypertensive effects of irbesartan were examined in 7 major placebo-controlled, 8 to 12-week trials in patients with baseline diastolic blood pressures of 95 to 110 mmHg. Doses of 1 to 900 mg were included in these trials in order to fully explore the dose-range of irbesartan. These studies allowed a comparison of once or twice-daily regimens at 150 mg/day, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Two of the 7 placebo-controlled trials identified above and 2 additional placebo-controlled studies examined the antihypertensive effects of irbesartan and hydrochlorothiazide in combination.
The 7 studies of irbesartan monotherapy included a total of 1915 patients randomized to irbesartan (1 to 900 mg) and 611 patients randomized to placebo. Once-daily doses of 150 to 300 mg provided statistically and clinically significant decreases in systolic and diastolic blood pressure with trough (24-hour post dose) effects after 6 to 12 weeks of treatment compared to placebo, of about 8 to 10/5 to 6 mmHg and 8 to 12/5 to 8 mmHg, respectively. No further increase in effect was seen at dosages greater than 300 mg. The dose-response relationships for effects on systolic and diastolic pressure are shown in Figures 3 and 4.
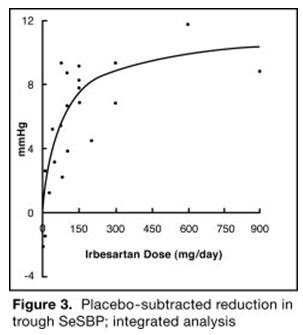
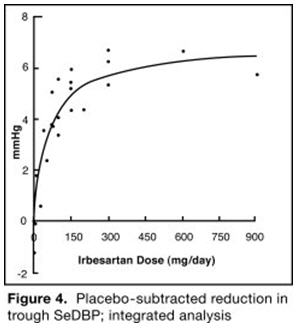
Once-daily administration of therapeutic doses of irbesartan gave peak effects at around 3 to 6 hours and, in one continuous ambulatory blood pressure monitoring study, again around 14 hours. This was seen with both once-daily and twice-daily dosing. Trough-to-peak ratios for systolic and diastolic response were generally between 60% and 70%. In a continuous ambulatory blood pressure monitoring study, once-daily dosing with 150 mg gave trough and mean 24-hour responses similar to those observed in patients receiving twice-daily dosing at the same total daily dose.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65 years of age, had generally similar responses. Irbesartan was effective in reducing blood pressure regardless of race, although the effect was somewhat less in blacks (usually a low-renin population). Black patients typically show an improved response with the addition of a low dose diuretic (e.g., 12.5 mg hydrochlorothiazide).
The effect of irbesartan is apparent after the first dose and is close to the full observed effect at 2 weeks. At the end of the 8-week exposure, about 2/3 of the antihypertensive effect was still present 1 week after the last dose. Rebound hypertension was not observed. There was essentially no change in average heart rate in irbesartan-treated patients in controlled trials.
14.2 Irbesartan-Hydrochlorothiazide
The antihypertensive effects of AVALIDE (irbesartan-hydrochlorothiazide) tablets were examined in 4 placebo-controlled studies in patients with mild-moderate hypertension (mean seated diastolic blood pressure [SeDBP] between 90 and 110 mmHg), one study in patients with moderate hypertension (mean seated systolic blood pressure [SeSBP] 160 to 179 mmHg or SeDBP 100 to 109 mmHg), and one study in patients with severe hypertension (mean SeDBP ≥110 mmHg) of 8 to 12 weeks. These trials included 3149 patients randomized to fixed doses of irbesartan (37.5 to 300 mg) and concomitant hydrochlorothiazide (6.25 to 25 mg).
Study I was a factorial study that compared all combinations of irbesartan (37.5 mg, 100 mg, and 300 mg or placebo) and hydrochlorothiazide (6.25 mg, 12.5 mg, and 25 mg or placebo).
Study II compared the irbesartan-hydrochlorothiazide combinations of 75/12.5 mg and 150/12.5 mg to their individual components and placebo.
Study III investigated the ambulatory blood pressure responses to irbesartan-hydrochlorothiazide (75/12.5 mg and 150/12.5 mg) and placebo after 8 weeks of dosing.
Study IV investigated the effects of the addition of irbesartan (75 or 150 mg) in patients not controlled (SeDBP 93–120 mmHg) on hydrochlorothiazide (25 mg) alone. In Studies I–III, the addition of irbesartan 150 to 300 mg to hydrochlorothiazide doses of 6.25, 12.5, or 25 mg produced further dose-related reductions in blood pressure at trough of 8 to 10 mmHg/3 to 6 mmHg, similar to those achieved with the same monotherapy dose of irbesartan. The addition of hydrochlorothiazide to irbesartan produced further dose-related reductions in blood pressure at trough (24 hours post dose) of 5 to 6/2 to 3 mmHg (12.5 mg) and 7 to 11/4 to 5 mmHg (25 mg), also similar to effects achieved with hydrochlorothiazide alone. Once-daily dosing with 150 mg irbesartan and 12.5 mg hydrochlorothiazide, 300 mg irbesartan and 12.5 mg hydrochlorothiazide, or 300 mg irbesartan and 25 mg hydrochlorothiazide produced mean placebo-adjusted blood pressure reductions at trough (24 hours post dosing) of about 13 to 15/7 to 9 mmHg, 14/9 to 12 mmHg, and 19 to 21/11 to 12 mmHg, respectively. Peak effects occurred at 3 to 6 hours, with the trough-to-peak ratios >65%.
In Study IV, the addition of irbesartan (75–150 mg) gave an additive effect (systolic/diastolic) at trough (24 hours post dosing) of 11/7 mmHg.
Initial Therapy
Studies V and VI had no placebo group, so effects described below are not all attributable to irbesartan or HCTZ.
Study V was conducted in patients with a mean baseline blood pressure of 162/98 mmHg and compared the change from baseline in SeSBP at 8 weeks between the combination group (irbesartan and HCTZ 150/12.5 mg), to irbesartan (150 mg) and to HCTZ (12.5 mg). These initial study regimens were increased at 2 weeks to AVALIDE 300/25 mg, irbesartan 300 mg, or to HCTZ 25 mg, respectively.
Mean reductions from baseline for SeDBP and SeSBP at trough were 14.6 mmHg and 27.1 mmHg for patients treated with AVALIDE, 11.6 mmHg and 22.1 mmHg for patients treated with irbesartan, and 7.3 mmHg and 15.7 mmHg for patients treated with HCTZ at 8 weeks, respectively. For patients treated with AVALIDE, the mean change from baseline in SeDBP was 3.0 mmHg lower (p=0.0013) and the mean change from baseline in SeSBP was 5.0 mmHg lower (p=0.0016) compared to patients treated with irbesartan, and 7.4 mmHg lower (p<0.0001) and 11.3 mmHg lower (p<0.0001) compared to patients treated with HCTZ, respectively. Withdrawal rates were 3.8% on irbesartan, 4.8% on HCTZ, and 6.7% on AVALIDE.
Study VI was conducted in patients with a mean baseline blood pressure of 172/113 mmHg and compared trough SeDBP at 5 weeks between the combination group (irbesartan and HCTZ 150/12.5 mg) and irbesartan (150 mg). These initial study regimens were increased at 1 week to AVALIDE 300/25 mg or to irbesartan 300 mg, respectively.
At 5 weeks, mean reductions from baseline for SeDBP and SeSBP at trough were 24.0 mmHg and 30.8 mmHg for patients treated with AVALIDE and 19.3 mmHg and 21.1 mmHg for patients treated with irbesartan, respectively. The mean SeDBP was 4.7 mmHg lower (p<0.0001) and the mean SeSBP was 9.7 mmHg lower (p<0.0001) in the group treated with AVALIDE than in the group treated with irbesartan. Patients treated with AVALIDE achieved more rapid blood pressure control with significantly lower SeDBP and SeSBP and greater blood pressure control at every assessment (Week 1, Week 3, Week 5, and Week 7). Maximum effects were seen at Week 7.
Withdrawal rates were 2.2% on irbesartan and 2.1% on AVALIDE.
In Studies I–VI, there was no difference in response for men and women or in patients over or under 65 years of age. Black patients had a larger response to hydrochlorothiazide than non-black patients and a smaller response to irbesartan. The overall response to the combination was similar for black and non-black patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
AVALIDE® (irbesartan-hydrochlorothiazide) film-coated tablets have markings on both sides and are available in the strengths and packages listed in the following table:
Tablet Strength
(irbesartan and hydrochlorothiazide)Film-Coated Tablet
Color/ShapeTablet
MarkingsPackage Size NDC Code 150 mg/12.5 mg peach, biconvex, oval-shaped heart debossed on one side and "2875" on the reverse Bottles of 30 0024-5855-30 300 mg/12.5 mg peach, biconvex, oval-shaped heart debossed on one side and "2876" on the reverse Bottles of 30 0024-5856-30 -
17 PATIENT COUNSELING INFORMATION
Pregnancy
Tell female patients of childbearing age about the consequences of exposure to AVALIDE during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physician as soon as possible.
Symptomatic Hypotension
Tell patients using AVALIDE that they may feel lightheaded, especially during the first days of use. Tell patients to inform their physician if they feel lightheaded or faint. Tell the patient, if fainting occurs, stop using AVALIDE and contact the prescribing doctor.
Tell patients using AVALIDE that getting dehydrated can lower their blood pressure too much and lead to lightheadedness and possible fainting. Dehydration may occur with excessive sweating, diarrhea, or vomiting and with not drinking enough liquids.
Potassium Supplements
Advise patients not to use potassium supplements or salt substitutes containing potassium without consulting their healthcare provider [see Drug Interactions (7.3)].
Acute Myopia and Secondary Angle-Closure Glaucoma
Advise patients to discontinue AVALIDE and seek immediate medical attention if they experience symptoms of Acute Myopia or Secondary Angle-Closure Glaucoma [see Warnings and Precautions (5.8)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 150/12.5 mg Tablet Bottle Label
30 Tablets
NDC: 0024-5855-30Avalide®
(irbesartan-
hydrochlorothiazide)
Tablets
150/12.5 mgRx only
SANOFI
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 300/12.5 mg Tablet Bottle Label
30 Tablets
NDC: 0024-5856-30Avalide®
(irbesartan-
hydrochlorothiazide)
Tablets
300/12.5 mgRx only
SANOFI
-
INGREDIENTS AND APPEARANCE
AVALIDE
irbesartan and hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0024-5855 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IRBESARTAN (UNII: J0E2756Z7N) (IRBESARTAN - UNII:J0E2756Z7N) IRBESARTAN 150 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color ORANGE (peach) Score no score Shape OVAL (biconvex) Size 13mm Flavor Imprint Code heart;2875 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0024-5855-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2013 2 NDC: 0024-5855-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2013 12/31/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020758 01/01/2013 AVALIDE
irbesartan and hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0024-5856 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IRBESARTAN (UNII: J0E2756Z7N) (IRBESARTAN - UNII:J0E2756Z7N) IRBESARTAN 300 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color ORANGE (peach) Score no score Shape OVAL (biconvex) Size 16mm Flavor Imprint Code heart;2876 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0024-5856-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2013 2 NDC: 0024-5856-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2013 08/31/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020758 01/01/2013 Labeler - Sanofi-Aventis U.S. LLC (824676584) Establishment Name Address ID/FEI Business Operations Sanofi Winthrop Industrie 763683216 ANALYSIS(0024-5855, 0024-5856) , LABEL(0024-5855, 0024-5856) , MANUFACTURE(0024-5855, 0024-5856) , PACK(0024-5855, 0024-5856) Establishment Name Address ID/FEI Business Operations Sanofi Winthrop Industrie 571879985 LABEL(0024-5855, 0024-5856) , PACK(0024-5855, 0024-5856) Establishment Name Address ID/FEI Business Operations Sanofi Chimie 262699775 ANALYSIS(0024-5855, 0024-5856) , API MANUFACTURE(0024-5855, 0024-5856) Establishment Name Address ID/FEI Business Operations Chinoin Pharmaceutical and Chemical Works Private Co., Ltd. 643939754 ANALYSIS(0024-5855, 0024-5856) , API MANUFACTURE(0024-5855, 0024-5856) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories, Inc 069777290 ANALYSIS(0024-5855, 0024-5856) Establishment Name Address ID/FEI Business Operations CAMBREX PROFARMACO MILANO S.R.L. 438051401 ANALYSIS(0024-5855, 0024-5856) , API MANUFACTURE(0024-5855, 0024-5856)
Trademark Results [Avalide]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AVALIDE 78260376 3068965 Dead/Cancelled |
SANOFI 2003-06-10 |
 AVALIDE 75375830 2312337 Live/Registered |
SANOFI 1997-10-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.