Rugby®Polyvinyl Alcohol w/Povidon1.4-0.6%
Polyvinyl Alcohol w/Povidon1.4-0.6% by
Drug Labeling and Warnings
Polyvinyl Alcohol w/Povidon1.4-0.6% by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
POLYVINYL ALCOHOL W/POVIDON1.4-0.6%- polyvinyl alcohol, povidon liquid
Rugby Laboratories
----------
Rugby®Polyvinyl Alcohol w/Povidon1.4-0.6%
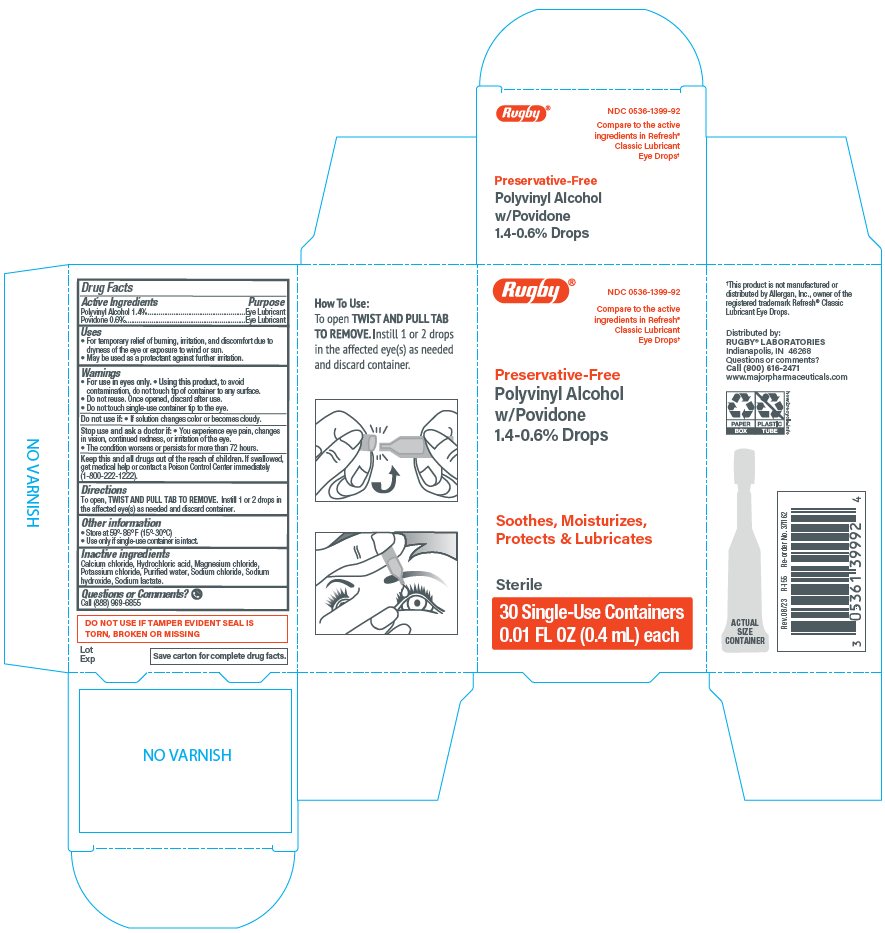
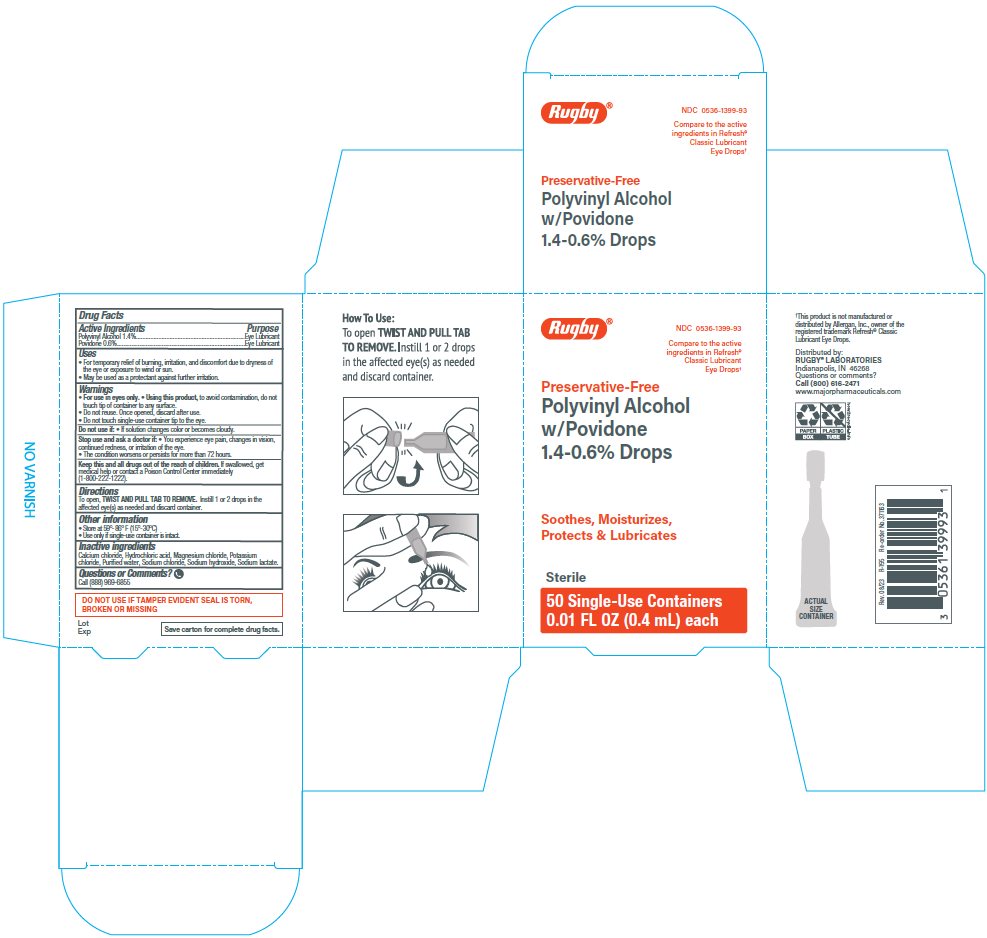
Uses
- ▪ For the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
- ▪ May be used as a protectant against further irritation.
Warnings
For use in eyes only.
Using this product, to avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard after use.
Do not touch single-use container tip to the eye.
Directions
To open, TWIST AND PULL TAB TO REMOVE. Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
Inactive ingredients
Calcium chloride, Hydrochloric acid, Magnesium chloride, Potassium chloride, Purified water, Sodium chloride, Sodium hydroxide, Sodium lactate.
Do not use
If solution changes color or becomes cloudy.
DO NOT USE IF TAMPER EVIDENT SEAL IS TORN, BROKEN OR MISSING
Save carton for complete drug facts.
*This product is not manufactured or distributed by Allergan, Inc., owner of the registered trademark Refresh® Classic Lubricant Eye Drops.
Distributed by:
RUGBY LABORATORIES
Indianapolis, IN 46268
Questions or comments?
Call (800) 616-2471
|
Rev. 08/23 |
R-155 |
Re-order No. 371162 |
|
Rev. 08/23 |
R-155 |
Re-order No. 371163 |
Principal Display Panel
Rugby®
NDC: 0536-1399-92
Compare to the active
ingredients in Refresh®
Classic Lubricant
Eye Drops*
Preservative-Free
Polyvinyl Alcohol
w/Povidone
1.4-0.6% Drops
Soothes, Moisturizes,
Protects & Lubricates
Sterile
30 Single-Use Containers
0.01 FL OZ (0.4 mL) each
Principal Display Panel
Rugby®
NDC: 0536-1399-93
Compare to the active
ingredients in Refresh®
Classic Lubricant
Eye Drops*
Preservative-Free
Polyvinyl Alcohol
w/Povidone
1.4-0.6% Drops
Soothes, Moisturizes,
Protects & Lubricates
Sterile
50 Single-Use Containers
0.01 FL OZ (0.4 mL) each
| POLYVINYL ALCOHOL W/POVIDON1.4-0.6%
polyvinyl alcohol, povidon liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.