ATORVASTATIN CALCIUM tablet, film coated
ATORVASTATIN CALCIUM by
Drug Labeling and Warnings
ATORVASTATIN CALCIUM by is a Prescription medication manufactured, distributed, or labeled by Northwind Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
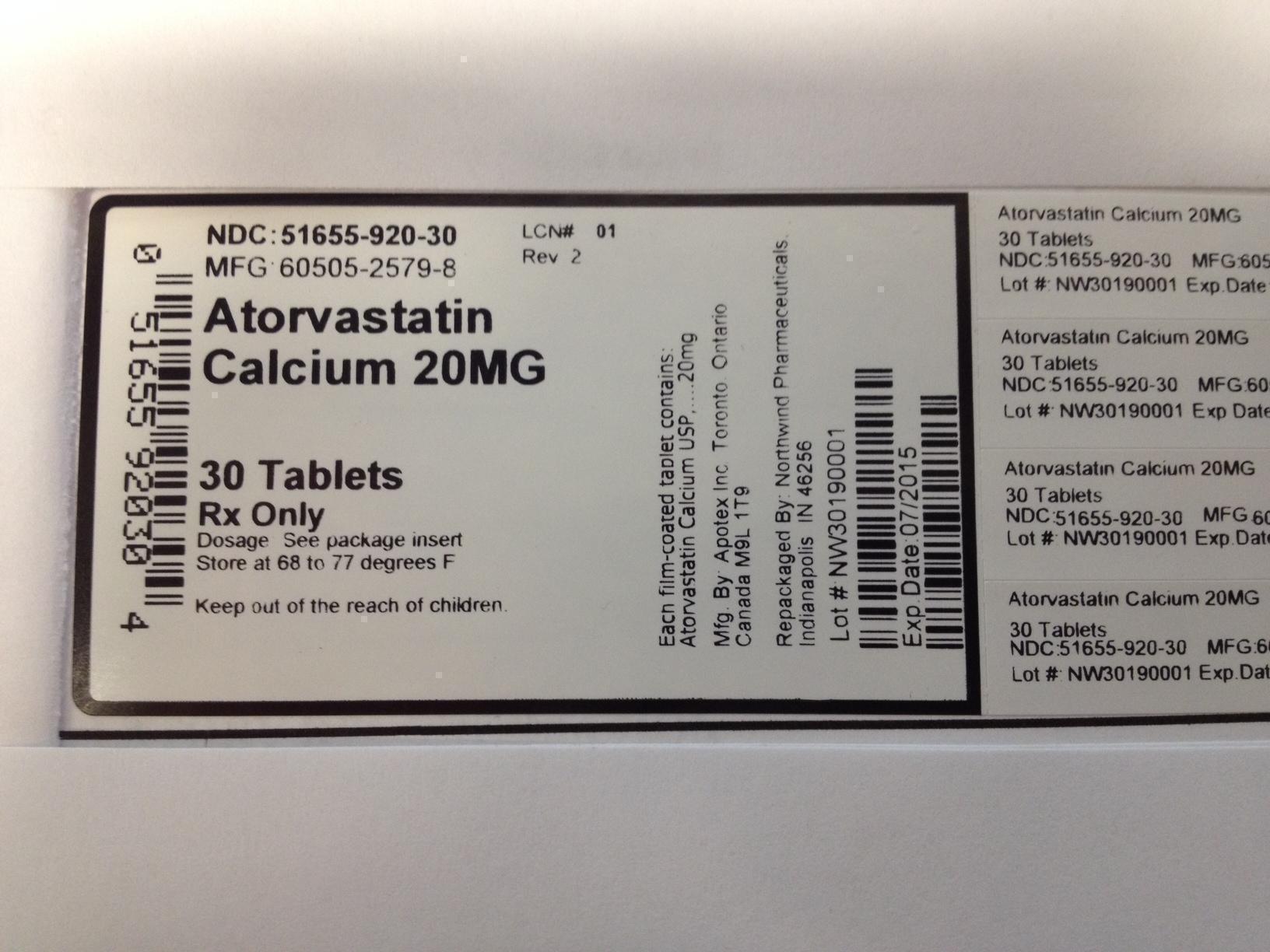
PRINCIPAL DISPLAY PANEL
NDC: 51655-920-30
MFG: 60505-2579-8
ATORVASTATIN CALCIUM 20 MG
30 TABLETS
RX ONLY
Dosage: See package insert
Store at 68 to 77 degrees F.
Keep out of reach of children.
Each film coated tablet contains Atorvastatin Calcium USP 20 MG
Mfg by: Apotex Inc. Toronto Ontario Canada, M9L 1T9
Repackaged by Northwind Pharmaceuticals Indianapolis, IN 46256
Lot # NW30190001 EXP Date: 07/2015
- WARNINGS AND PRECAUTIONS
-
INGREDIENTS AND APPEARANCE
ATORVASTATIN CALCIUM
atorvastatin calcium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51655-920(NDC:60505-2579) Route of Administration Oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATORVASTATIN CALCIUM (UNII: 48A5M73Z4Q) (ATORVASTATIN - UNII:A0JWA85V8F) ATORVASTATIN 20 mg in 30 Product Characteristics Color white Score no score Shape OVAL Size 11mm Flavor Imprint Code APO;ATV20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51655-920-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076477 03/11/2014 Labeler - Northwind Pharmaceuticals (036986393) Registrant - Northwind Pharmaceuticals (036986393) Establishment Name Address ID/FEI Business Operations Northwind Pharmaceuticals 036986393 repack(51655-920)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.