FACOL COLD AND FLU DAY- acetaminophen, guaifenesin, phenylephrine hcl, dextromethorphan hydrobromide capsule, liquid filled
Facol Cold and Flu Day by
Drug Labeling and Warnings
Facol Cold and Flu Day by is a Otc medication manufactured, distributed, or labeled by JW Holdings, RP Bio Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Do not use
■ For children who are younger than 3 months old. (new born baby)
■ If you ever had an allergic reaction to this product or any of its ingredients.
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
■ If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease) or 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or a pharmacist) - Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
■ nervousness, dizziness, or sleeplessness, constipation occurs
■ redness or swelling is present ■ fever get worse or lasts more than 3 days
■ new severe symptoms occur such as shock (anaphylaxis), Stevens Johnson Syndrome, Lyell Syndrome, Asthma, Dyshepatia or Interstitial lung diseases.
■ pain, cough, nasal congestion gets worse or lasts after 5-6 times of taking a dose. - If Pregnant or breast-feeding
- Keep out of reach of children.
- Overdose warning:
- Uses
- Other Information
- Questions or Comments?
- Inactive Ingredients
- Warnings
-
Directions
■ Do not take more than directed (see overdose warning)
■ adults and children 15 years and older: take 2 capsules after a meal 2 times a day
■ swallow whole-do not crush, chew or dissolve
■ do not take night time capsule in less than 6 hours after taking day time capsule
■ children under 15 years: do not use and ask a doctor. - INDICATIONS & USAGE
- Active Ingredient(in each tablet) Purpose
-
PRINCILA DISPLAY PANEL
NDC: 69365-001-01
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
MULTI
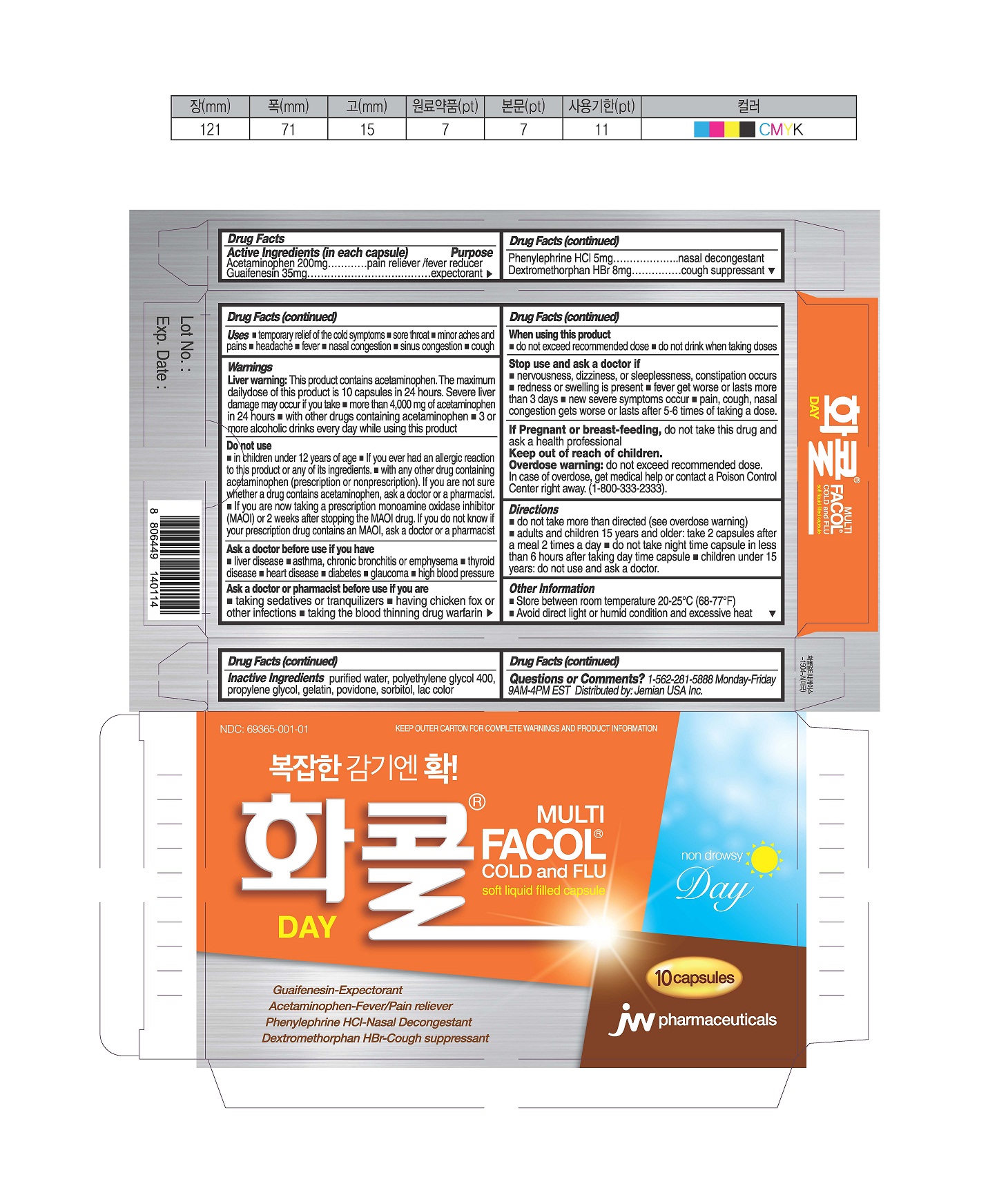
Facol® Cold and Flu
soft liquid filled capsule
DAY
Guaifenesin-Expectorant
Acetaminophen-Fever/Pain Reducer
Phenylephrine HCl-Nasal Decongestant
Dextromethorphan HBr-Cough Suppressant
10 capsules
JW pharmaceuticals
-
INGREDIENTS AND APPEARANCE
FACOL COLD AND FLU DAY
acetaminophen, guaifenesin, phenylephrine hcl, dextromethorphan hydrobromide capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69365-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 200 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 35 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 8 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 40 mg POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 459 mg PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 40 mg GELATIN (UNII: 2G86QN327L) 203.93 mg POVIDONE K29/32 (UNII: 390RMW2PEQ) 33 mg SORBITOL (UNII: 506T60A25R) 141.1 mg COCHINEAL (UNII: TZ8Z31B35M) 2.185 mg ALUMINUM OXIDE (UNII: LMI26O6933) 2.185 mg Product Characteristics Color yellow Score no score Shape CAPSULE Size 20mm Flavor Imprint Code FacD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69365-001-02 1 in 1 KIT 08/01/2015 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 08/01/2015 Labeler - JW Holdings (631094492) Registrant - JW Holdings (631094492) Establishment Name Address ID/FEI Business Operations RP Bio Inc. 689851235 manufacture(69365-001) , pack(69365-001) , analysis(69365-001) , label(69365-001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.