Phenylbutazone by MWI/VetOne PHENYLBUTAZONE powder
Phenylbutazone by
Drug Labeling and Warnings
Phenylbutazone by is a Animal medication manufactured, distributed, or labeled by MWI/VetOne. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
For Horses Only: Administer orally (using the 0.6 ounce (18 mL) scoop provided) on a small amount of palatable feed and mix well. Give 1 to 2 level scoops per 500 pounds of body weight, but do not exceed 4 scoops per animal daily. Use the high dose for the first 48 hours, then gradually reduce to a maintenance dose.
- CONTRAINDICATIONS
- WARNING
- HUMAN WARNING
- PRECAUTION
-
CLINICAL PHARMACOLOGY
Phenylbutazone was first synthesized in 1948 and introduced into human medicine in 1949. Kuzell (1), (2), (3), Payne (4), Fleming (5) and Denko (6) demonstrated the clinical effectiveness of phenylbutazone in gout, gouty arthritis, acute arthritis, acute rheumatism and various other rheumatoid disorders in humans. Fabre (7), Domenjoz (8), Wilhelmi (9) and Yourish (10) have established the anti-rheumatic and anti-inflammatory activity of phenylbutazone. It is entirely unrelated to the steroid hormones.
Toxicity of phenylbutazone has been investigated in rats and mice (11) and dogs (12).
Phenylbutazone has been used by Camberos (13) in thoroughbred horses. Favorable results were reported in cases of traumatism, muscle rupture, strains and inflammations of the third phalanx. Results were not as favorable in the period treatment of osteoarthritis of the stifle and hip, arthrosis of the trapezious muscles and general arthritis. Sutter (14) reported a favorable response in chronic equine arthritis of long duration, fair results in severely bruised mare and poor results in two cases where the condition was limited to the third phalanx. - HOW SUPPLIED
-
References
1. Kuzell, WC, Schaffarzick, RW, Naughler, WE, Gandia, C, and Mankle, EA: A.M.A. Arch. Inst. Med., 92:646 (1953).
2. Kuzell, WC, Schaffarzick, RW, Brown, B, and Mankle, EA: J.A.M.A., 149:729 (1952).
3. Kuzell, WC, and Schaffarzick, RW: Calif. Med., 77:319 (1952).
4. Payne, RW, Shelter, MR, Farr, CH, Hellbaum, AA, and Ishmall, WK: J. Lab. Clin. Med., 45:331 (1955).
5. Fleming, J, and Will, G: Ann. Rheumat., Dis., 12:95 (1953).
6. Denko, CW, and Rumi, D: American Pract., 6:1865 (1955).
7. Fabre, J, et al: Semain. Hop. (Paris), 31:87 (1955).
8. Domenjoz, R, et al: Arzneimittel-Forsch, 5:488 (1955).
9. Wilhelmi, G, and Pulver, R: Arzneimittel-Forsch, 5:221 (1955).
10. Yourish, W, Paton, B, Brodie, B, and Burns, J: A.M.A. Arch. Ophth., 53:264 (1955).
11. Hazelton, LW, Tusing, TW, and Hollana, EG: J. Pharmacol. Exper. Ther., 109:387 (1953).
12. Ogilvie, FB, and Sutter, MD: Vet. Med. 52:492 (1957).
13. Camberos, HR: Rev. Med. Vet. (Buenos Aries), 38:9 (1956).
14. Sutter, MD: Vet. Med., 53:83 (1958).Approved by FDA under ANADA # 200-334
-
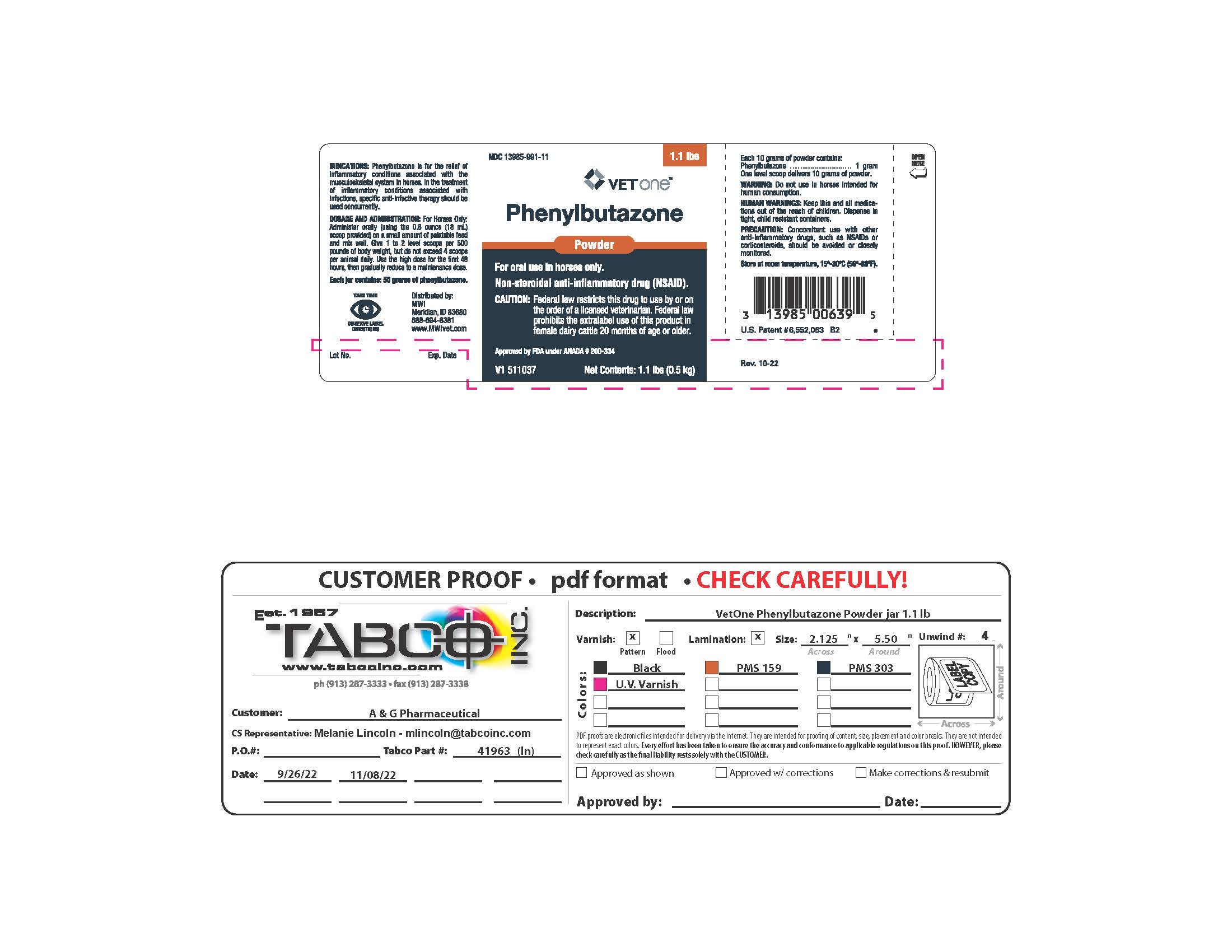
Principal Display Panel - Phenylbutazone Powder (1.1 lb jar)
NDC: 13985-991-11
VETONE
Phenylbutazone
Powder
For Oral Use In Horses Only
NON-STEROIDAL ANTI-INFLAMMATORY DRUG (NSAID)
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits the extralabel use of this product in female dairy cattle 20 months of age or older.
Approved by FDA under ANADA # 200-334
Net Contents: 1.1 lb (0.5 kg)

-
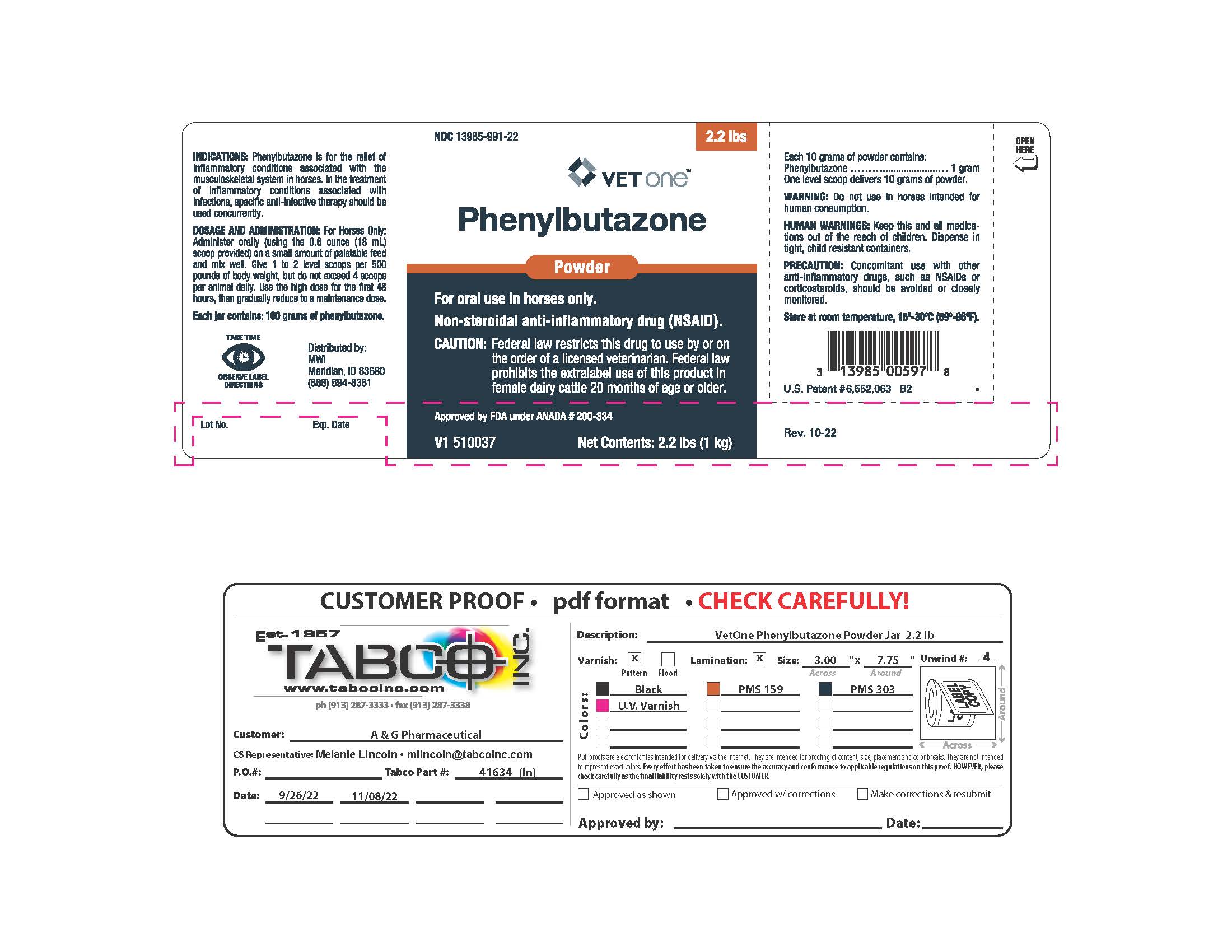
Principal Display Panel - Phenylbutazone Powder (2.2 lb jar)
NDC: 13985-991-22
VETONE™
Phenylbutazone
Powder
For Oral Use In Horses Only
NON-STEROIDAL ANTI-INFLAMMATORY DRUG (NSAID).
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Federal law prohibits the extralabel use of this product in female dairy cattle 20 months of age or older.
Approved by FDA under ANADA # 200-334
Net Contents: 2.2 lb (1 kg)

-
INGREDIENTS AND APPEARANCE
PHENYLBUTAZONE
phenylbutazone powderProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-991 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLBUTAZONE (UNII: GN5P7K3T8S) (PHENYLBUTAZONE - UNII:GN5P7K3T8S) PHENYLBUTAZONE 1 g in 10 g Product Characteristics Color orange (Light orange powder) Score Shape Size Flavor ORANGE (ORANGE FLAVOR) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-991-11 500 g in 1 JAR 2 NDC: 13985-991-22 1000 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200334 05/01/2009 01/10/2025 Labeler - MWI/VetOne (019926120)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.