UNDA 17- jateorhiza palmata, boldo leaf, rhamnus frangula, scabiosa succisa underground parts, valeriana officinalis, dulcamara, rumex crispus, argentum metallicum liquid
Unda 17 by
Drug Labeling and Warnings
Unda 17 by is a Homeopathic medication manufactured, distributed, or labeled by Seroyal USA, SAN’UP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Boldo Leaf 4X
Dulcamara (Bittersweet) Shoots 4X
Jateorhiza palmata (Columbo) Root 4X
Rhamnus frangula (Buckthorn) Bark 4X
Rumex crispus (Curly dock) Underground Parts 4X
Scabiosa succisa (Devil's Bit) Underground Parts 4X
Valeriana officinalis (Valerian) Root 4X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptom sassociated with
mild skin irritationpoor digestion
Directions
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- OTHER SAFETY INFORMATION
-
PRINCIPAL DISPLAY PANEL
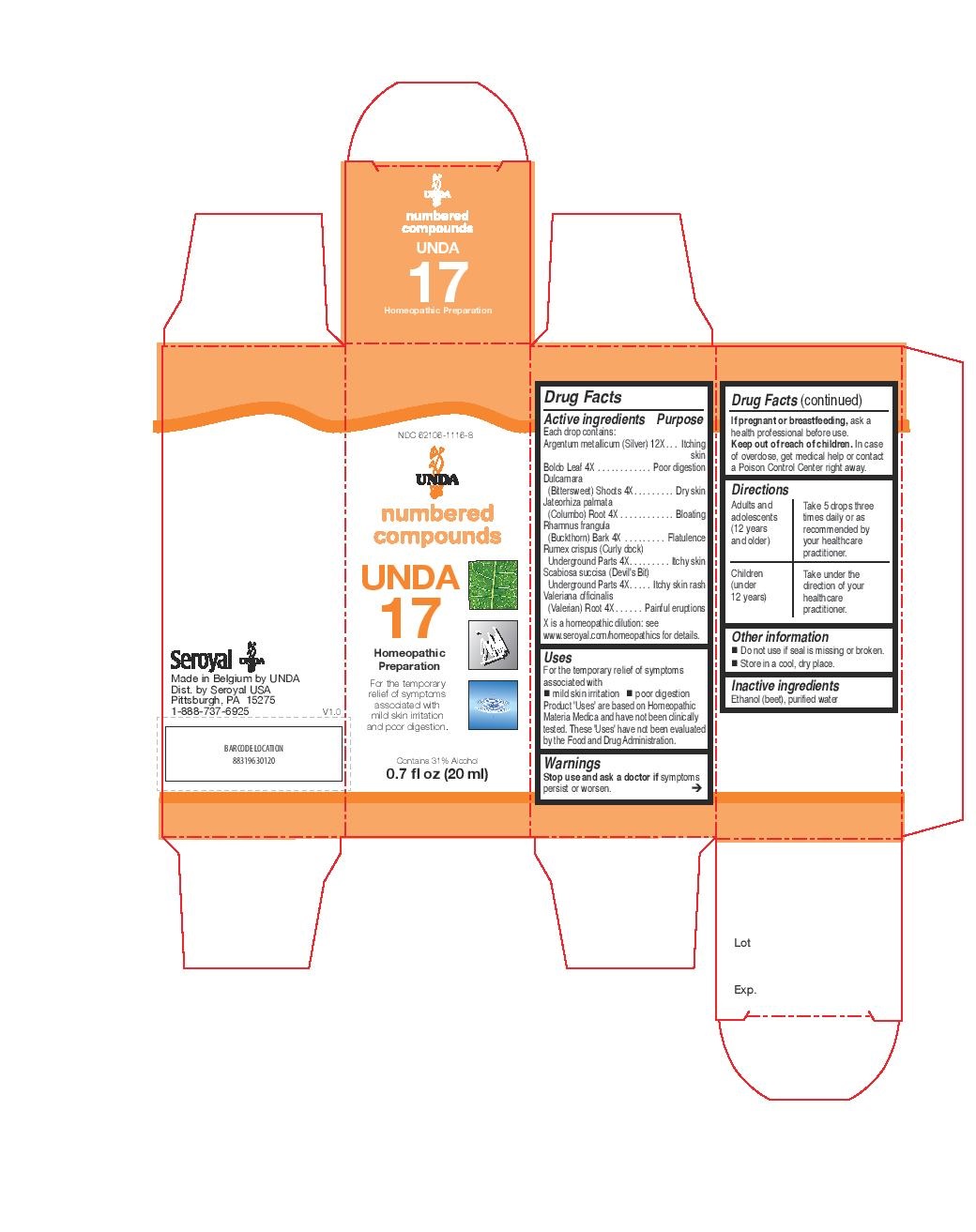
NDC: 62106-1116-8
UNDA
numbered compounds
UNDA 17
Homeopathic Preparation
For the temporary relief of symptoms
associated with mild skin irritation
and poor digestion.
Contains 31% Alcohol
0.7 fl oz (20 ml) -
INGREDIENTS AND APPEARANCE
UNDA 17
jateorhiza palmata, boldo leaf, rhamnus frangula, scabiosa succisa underground parts, valeriana officinalis, dulcamara, rumex crispus, argentum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62106-1116 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength JATEORHIZA CALUMBA ROOT (UNII: V36I2B8LD5) (JATEORHIZA CALUMBA ROOT - UNII:V36I2B8LD5) JATEORHIZA CALUMBA ROOT 4 [hp_X] in 20 mL PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL FRANGULA ALNUS BARK (UNII: S2D77IH61R) (FRANGULA ALNUS BARK - UNII:S2D77IH61R) FRANGULA ALNUS BARK 4 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 4 [hp_X] in 20 mL CHAMAELIRIUM LUTEUM ROOT (UNII: DQV54Y5H3U) (CHAMAELIRIUM LUTEUM ROOT - UNII:DQV54Y5H3U) CHAMAELIRIUM LUTEUM ROOT 4 [hp_X] in 20 mL SOLANUM DULCAMARA STEM (UNII: IR986LE7DF) (SOLANUM DULCAMARA STEM - UNII:IR986LE7DF) SOLANUM DULCAMARA STEM 4 [hp_X] in 20 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 4 [hp_X] in 20 mL SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62106-1116-8 1 in 1 CARTON 02/06/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/06/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1116)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.