SAFEHANDS HAND SANITIZER FRAGRANCE FREE- benzalkonium chloride liquid
safeHands Hand Sanitizer by
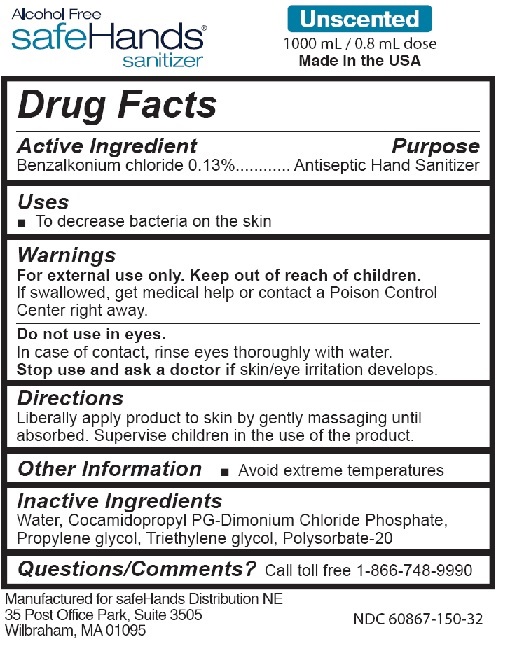
Drug Labeling and Warnings
safeHands Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Safehands Distribution Ne, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
-
Package Labeling
safeHands®
Hand Sanitizerkills 99.9% of common germs
FRAGRANCE FREE
MOISTURIZING & FOAMING
ALCOHOL-FREE FORMULA
1.75 fl oz (52 mL)
NDC: 60867-150-02
Manufactured for
safeHands Distribution NE
35 Post Oak Office Park, Suite 3505
Wilbraham, MA 01095
Call toll free
1-866-748-9990
SAFEHANDS.COM
1.75 fl oz


7 fl oz


18 fl oz


1000 mL

128 fl oz


res
-
INGREDIENTS AND APPEARANCE
SAFEHANDS HAND SANITIZER FRAGRANCE FREE
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60867-150 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRIETHYLENE GLYCOL (UNII: 3P5SU53360) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60867-150-02 52 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/12/2021 2 NDC: 60867-150-07 200 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/12/2021 3 NDC: 60867-150-18 500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/12/2021 4 NDC: 60867-150-32 1000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/12/2021 5 NDC: 60867-150-99 3785 mL in 1 JUG; Type 0: Not a Combination Product 05/12/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/12/2021 Labeler - Safehands Distribution Ne, LLC (080026877)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.