KEYSTONE- triclosan solution
Keystone by
Drug Labeling and Warnings
Keystone by is a Otc medication manufactured, distributed, or labeled by Ecolab Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
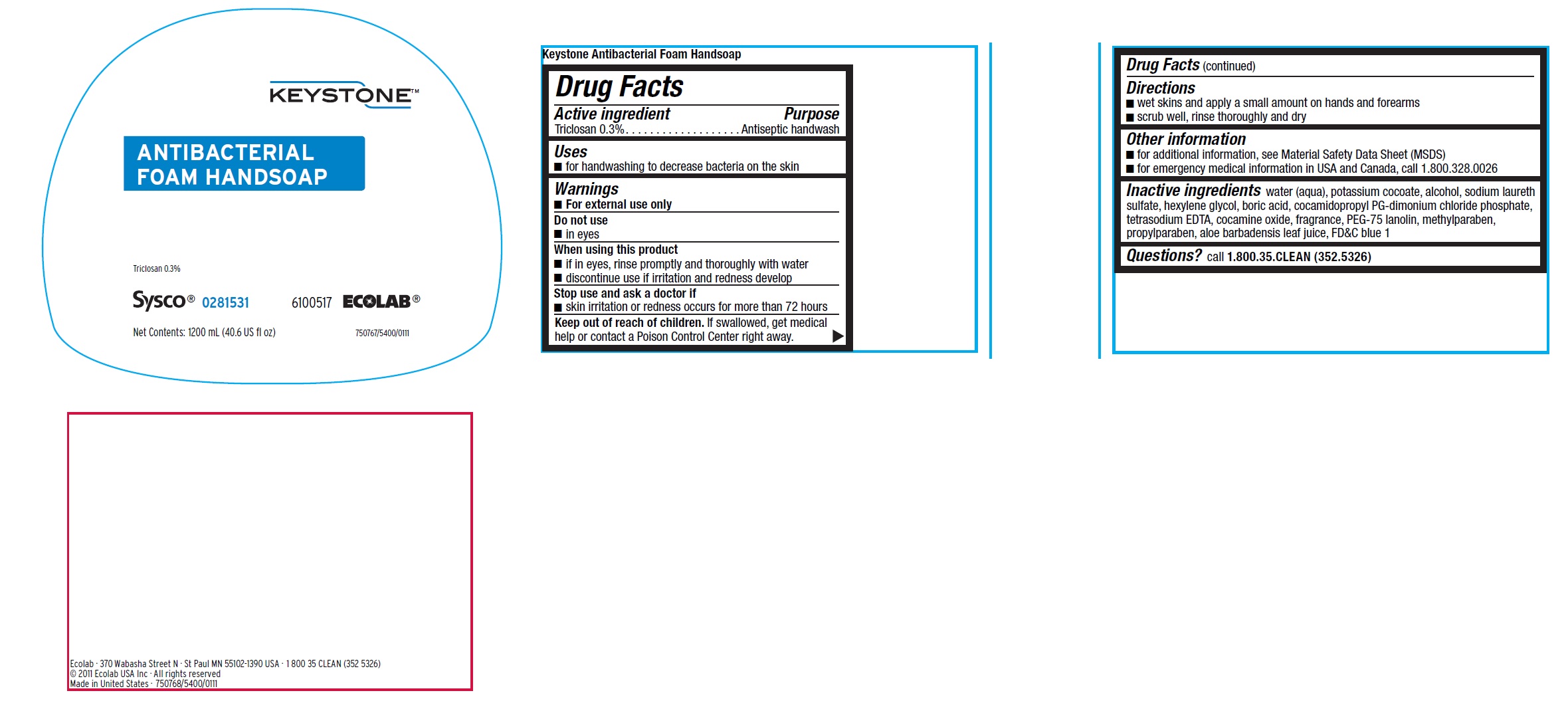
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
-
Principal display panel and representative label
KEYSTONE
ANTIBACTERIAL FOAM HANDSOAP
Triclosan 0.3%
SYSCO 0281531
6100517 ECOLAB
Net Contents: 1200 mL (40.6 US fl oz)
750767/5400/0111
Ecolab · 370 Wabasha Street N · St Paul MN 55102-1390 USA · 1 800 35 CLEAN (352 5326)
© 2011 Ecolab USA Inc · All rights reserved
Made in United States · 750768/5400/0111
-
INGREDIENTS AND APPEARANCE
KEYSTONE
triclosan solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 47593-341 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRICLOSAN (UNII: 4NM5039Y5X) (TRICLOSAN - UNII:4NM5039Y5X) TRICLOSAN 0.3 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) ALCOHOL (UNII: 3K9958V90M) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) HEXYLENE GLYCOL (UNII: KEH0A3F75J) BORIC ACID (UNII: R57ZHV85D4) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) EDETATE SODIUM (UNII: MP1J8420LU) COCAMINE OXIDE (UNII: QWA2IZI6FI) PEG-75 LANOLIN (UNII: 09179OX7TB) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47593-341-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/26/2000 2 NDC: 47593-341-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/26/2000 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 07/26/2000 Labeler - Ecolab Inc. (006154611)
Trademark Results [Keystone]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.