ONUREG- azacitidine tablet, film coated
ONUREG by
Drug Labeling and Warnings
ONUREG by is a Prescription medication manufactured, distributed, or labeled by Celgene Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ONUREG safely and effectively. See full prescribing information for ONUREG.
ONUREG (azacitidine) tablets, for oral use
Initial U.S. Approval: 2004INDICATIONS AND USAGE
ONUREG is a nucleoside metabolic inhibitor indicated for continued treatment of adult patients with acute myeloid leukemia who achieved first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) following intensive induction chemotherapy and are not able to complete intensive curative therapy (1).
DOSAGE AND ADMINISTRATION
- Do not substitute ONUREG for intravenous or subcutaneous azacitidine. The indications and dosing regimen for ONUREG differ from that of intravenous or subcutaneous azacitidine (2.1, 5.1).
- Administer ONUREG 300 mg orally once daily on Days 1 through 14 of each 28-day cycle (2.2).
- Administer an antiemetic before each dose for at least the first 2 cycles (2.2).
DOSAGE FORMS AND STRENGTHS
Tablets: 200 mg and 300 mg (3).
CONTRAINDICATIONS
History of severe hypersensitivity to azacitidine or its components (4).
WARNINGS AND PRECAUTIONS
- Risks of Substitution with Other Azacitidine Products: Do not substitute ONUREG for intravenous or subcutaneous azacitidine (2.1, 5.1).
- Myelosuppression: Monitor complete blood counts every other week for the first 2 cycles and prior to the start of each cycle thereafter. Increase monitoring to every other week for the 2 cycles after any dose reduction. Withhold and then resume at same or reduced dose or discontinue ONUREG based on severity (2.3, 5.2).
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and use of effective contraception (5.4, 8.1, 8.3).
ADVERSE REACTIONS
The most common adverse reactions (≥ 10%) are nausea, vomiting, diarrhea, fatigue/asthenia, constipation, pneumonia, abdominal pain, arthralgia, decreased appetite, febrile neutropenia, dizziness, and pain in extremity (6).
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb Company at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Recommended Dosage
2.3 Monitoring and Dosage Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks of Substitution with Other Azacitidine Products
5.2 Myelosuppression
5.3 Increased Early Mortality in Patients with Myelodysplastic Syndromes
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
Do not substitute ONUREG for intravenous or subcutaneous azacitidine. The indications and dosing regimen for ONUREG differ from that of intravenous or subcutaneous azacitidine [see Warnings and Precautions (5.1)].
2.2 Recommended Dosage
The recommended dosage of ONUREG is 300 mg orally once daily with or without food on Days 1 through 14 of each 28-day cycle. Continue ONUREG until disease progression or unacceptable toxicity.
Administer an antiemetic 30 minutes prior to each dose of ONUREG for the first 2 cycles. Antiemetic prophylaxis may be omitted after 2 cycles if there has been no nausea and vomiting.
If the absolute neutrophil count (ANC) is less than 0.5 Gi/L on Day 1 of a cycle, do not administer ONUREG. Delay the start of the cycle until the ANC is 0.5 Gi/L or more.
Instruct patients on the following:
- Swallow tablets whole. Do not cut, crush, or chew the tablets.
- Take a dose about the same time each day.
- If a dose of ONUREG is missed, or not taken at the usual time, take the dose as soon as possible on the same day, and resume the normal schedule the following day. Do not take 2 doses on the same day.
- If a dose is vomited, do not take another dose on the same day. Resume the normal schedule the following day.
ONUREG is a hazardous drug. Follow applicable special handling and disposal procedures.1
2.3 Monitoring and Dosage Modifications for Adverse Reactions
Monitor complete blood count every other week for the first 2 cycles and prior to the start of each cycle thereafter. Increase monitoring to every other week for the 2 cycles after any dose reduction for myelosuppression.
The recommended dosage modifications for adverse reactions are provided in Table 1.
Table 1: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity Recommended Dosage Modification Myelosuppression [see Warnings and Precautions (5.2)]
Neutrophils less than 0.5 Gi/L on Cycle Day 1
- Interrupt treatment. Resume at the same dose once neutrophils return to 0.5 Gi/L or higher.
Neutrophils less than 1 Gi/L with fever at anytime
First Occurrence
- Interrupt treatment. Resume at the same dose once neutrophils return to 1 Gi/L or higher.
Occurrence in 2 Consecutive Cycles
- Interrupt treatment. After neutrophils return to 1 Gi/L or higher, resume at reduced dose of 200 mg.
- If a patient continues to experience febrile neutropenia after dose reduction, reduce the treatment duration by 7 days.
- If febrile neutropenia reoccurs after dose and schedule reduction, discontinue ONUREG.
Platelets less than 50 Gi/L with bleeding
First Occurrence
- Interrupt dose. Resume at the same dose once platelets return to 50 Gi/L or higher.
Occurrence in 2 Consecutive Cycles
- Interrupt dose. After platelets return to 50 Gi/L or higher, resume at reduced dose of 200 mg.
- If a patient continues to experience thrombocytopenia with bleeding after dose reduction, reduce the treatment duration by 7 days.
- If thrombocytopenia with bleeding reoccurs after dose and schedule reduction, discontinue ONUREG.
Gastrointestinal Toxicity [see Adverse Reactions (6.1)]
Grade 3 or 4 Nausea or Vomiting
- Interrupt dose. Resume at the same dose once toxicity has resolved to Grade 1 or lower.
- If toxicity reoccurs, interrupt dose until resolved to Grade 1 or lower. Resume at reduced dose of 200 mg.
- If a patient continues to experience the toxicity after dose reduction, reduce the treatment duration by 7 days.
- If the toxicity continues or reoccurs after dose and schedule reduction, discontinue ONUREG.
Grade 3 or 4 Diarrhea
- Interrupt dose. Resume at the same dose once toxicity has resolved to Grade 1 or lower.
- If toxicity reoccurs, interrupt dose until resolved to Grade 1 or lower. Resume at reduced dose of 200 mg.
- If a patient continues to experience the toxicity after dose reduction, reduce the treatment duration by 7 days.
- If the toxicity continues or reoccurs after dose and schedule reduction, discontinue ONUREG.
Other Adverse Reactions [see Adverse Reactions (6.1)]
Grade 3 or 4
- Interrupt dose and provide medical support. Resume at the same dose once toxicity has resolved to Grade 1 or lower.
- If toxicity re-occurs, interrupt dose until resolved to Grade 1 or lower. Resume at reduced dose of 200 mg.
- If a patient continues to experience the toxicity after dose reduction, reduce the treatment duration by 7 days.
- If the toxicity continues or reoccurs after dose and schedule reduction, discontinue ONUREG.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ONUREG is contraindicated in patients with known severe hypersensitivity to azacitidine or its components [see Adverse Reactions (6.2), Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks of Substitution with Other Azacitidine Products
Due to substantial differences in the pharmacokinetic parameters [see Clinical Pharmacology (12.3)], the recommended dose and schedule for ONUREG are different from those for the intravenous or subcutaneous azacitidine products. Treatment of patients using intravenous or subcutaneous azacitidine at the recommended dosage of ONUREG may result in a fatal adverse reaction. Treatment of patients using ONUREG at the doses recommended for intravenous or subcutaneous azacitidine may not be effective.
Do not substitute ONUREG for intravenous or subcutaneous azacitidine [see Dosage and Administration (2.1)].
5.2 Myelosuppression
New or worsening Grade 3 or 4 neutropenia and thrombocytopenia occurred in 49% and 22% of patients who received ONUREG, respectively. Febrile neutropenia occurred in 12%. A dose reduction was required for 7% and 2% of patients due to neutropenia and thrombocytopenia, respectively. Less than 1% of patients discontinued ONUREG due to either neutropenia or thrombocytopenia.
Monitor complete blood counts and modify the dosage as recommended [see Dosage and Administration (2.2, 2.3)]. Provide standard supportive care, including hematopoietic growth factors, if myelosuppression occurs.
5.3 Increased Early Mortality in Patients with Myelodysplastic Syndromes
In AZA-MDS-003 (NCT01566695), 216 patients with red blood cell transfusion-dependent anemia and thrombocytopenia due to myelodysplastic syndromes were randomized to ONUREG or placebo. One-hundred and seven patients received a median of 5 cycles of ONUREG 300 mg daily for 21 days of a 28-day cycle. Enrollment was discontinued early due to a higher incidence of early fatal and/or serious adverse reactions in patients who received ONUREG compared with placebo. The most frequent fatal adverse reaction was sepsis. The safety and effectiveness of ONUREG for treatment of myelodysplastic syndromes have not been established. Treatment of patients with myelodysplastic syndromes with ONUREG is not recommended outside of controlled trials.
5.4 Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, ONUREG can cause fetal harm when administered to a pregnant woman. Azacitidine administered to pregnant rats via a single intraperitoneal dose less than the recommended human daily dose of oral azacitidine on a mg/m2 basis caused fetal death and anomalies.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ONUREG and for at least 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with ONUREG and for at least 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Acute Myeloid Leukemia
The safety of ONUREG was evaluated in QUAZAR [see Clinical Studies (14)]. Patients received ONUREG 300 mg (N=236) or placebo (N=233) orally once daily on Days 1 through 14 of each 28-day cycle. Among patients who received ONUREG, 71% were exposed for 6 months or longer, and 49% were exposed for greater than one year. The median duration of exposure to ONUREG was 11.6 months (range: 0.5 to 74.3 months) and the median number of cycles was 12 (range: 1 to 82 cycles).
Serious adverse reactions occurred in 15% of patients who received ONUREG. Serious adverse reactions in ≥ 2% of patients who received ONUREG were pneumonia (8%) and febrile neutropenia (7%). One fatal adverse reaction (sepsis) occurred in a patient who received ONUREG.
Permanent discontinuation of ONUREG due to an adverse reaction occurred in 8% of patients. Adverse reactions which resulted in permanent discontinuation of ONUREG in > 1% of patients included nausea (2.1%), diarrhea (1.7%), and vomiting (1.3%). Interruptions of ONUREG due to an adverse reaction occurred in 35% of patients. Adverse reactions which required an interruption of ONUREG in > 5% of patients included neutropenia (20%), thrombocytopenia (8%), and nausea (6%).
Dose reductions of ONUREG due to an adverse reaction occurred in 14% of patients. Adverse reactions which required a dose reduction in > 1% of patients included neutropenia (6%), diarrhea (3.4%), thrombocytopenia (1.7%), and nausea (1.7%).
The most common (≥ 10%) adverse reactions were nausea, vomiting, diarrhea, fatigue/asthenia, constipation, pneumonia, abdominal pain, arthralgia, decreased appetite, febrile neutropenia, dizziness, and pain in extremity.
Table 2 summarizes the adverse reactions in QUAZAR.
Table 2: Adverse Reactions (≥ 5%) in Patients with AML Who Received ONUREG with a Difference Between Arms of > 2% Compared to Placebo in QUAZAR Adverse Reaction ONUREG
(N=236)Placebo
(N=233)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)a Grouped term includes abdominal pain, abdominal pain upper, abdominal discomfort, and gastrointestinal pain.
b Grouped term includes fatigue and asthenia.
c Broad scope term includes influenza, pneumonia, respiratory tract infection, respiratory tract infection viral, bronchopulmonary aspergillosis, lung infection, Staphylococcal infection, atypical pneumonia, lower respiratory tract infection, lung abscess, Pneumocystis jirovecii pneumonia, pneumonia bacterial, pneumonia fungal, Pseudomonas infection, hemoptysis, productive cough, pleural effusion, atelectasis, pleuritic pain, rales, Enterobacter test positive, and Hemophilus test positive.Gastrointestinal disorders
Nausea
65
3
24
< 1
Vomiting
60
3
10
0
Diarrhea
50
5
21
1
Constipation
39
1
24
0
Abdominal paina
22
2
13
< 1
General disorders and administration site conditions
Fatigue / astheniab
44
4
25
1
Infections
Pneumoniac
27
9
17
5
Musculoskeletal and connective tissue disorders
Arthralgia
14
1
10
< 1
Pain in extremity
11
< 1
5
0
Metabolism and nutrition disorders
Decreased appetite
13
1
6
1
Blood and lymphatic disorders
Febrile neutropenia
12
11
8
8
Nervous system disorders
Dizziness
11
0
9
0
Clinically relevant adverse reactions that did not meet criteria for inclusion in Table 2 were weight decreased (4%) in patients who received ONUREG.
Neutropenia, thrombocytopenia, and anemia of any grade occurred in 74%, 65%, and 25% of patients who received ONUREG. Table 3 summarizes select Grades 3 or 4 hematological laboratory abnormalities in QUAZAR.
Table 3: Selected Hematological Laboratory Abnormalities That Worsened from Baseline in Patients Who Received ONUREG in QUAZAR ONUREG Placebo Laboratory Abnormality Baseline
Grade 0-2
NPost-Baseline
Grade 3 or 4
n (%)Baseline
Grade 0-2
NPost-Baseline
Grade 3 or 4
n (%)Neutropenia
223
109 (49)
217
50 (23)
Thrombocytopenia
222
46 (21)
212
22 (10)
Anemia
229
10 (4)
223
7 (3)
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of intravenous or subcutaneous azacitidine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reaction
- Interstitial lung disease
- Tumor lysis syndrome
- Sweet's syndrome (acute febrile neutrophilic dermatosis)
- Necrotizing fasciitis (including fatal cases)
- Differentiation syndrome
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [see Clinical Pharmacology (12.1)] and findings in animals, ONUREG can cause fetal harm when administered to a pregnant woman. There are no available data on ONUREG use in pregnant women to evaluate for a drug-associated risk. Azacitidine was teratogenic and caused embryo-fetal lethality in animals at doses less than the recommended human daily dose of oral azacitidine on a mg/m2 basis (see Data). Advise pregnant women of the potential risk to the fetus.
The estimated background of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No reproductive or developmental toxicity studies have been conducted with oral azacitidine.
Early embryotoxicity studies in mice revealed a 44% frequency of intrauterine embryonal death (increased resorption) after a single intraperitoneal injection of 6 mg/m2 azacitidine (at doses less than the recommended human daily dose of oral azacitidine on a mg/m2 basis) on gestation Day 10. Developmental abnormalities in the brain have been detected in mice given azacitidine on or before gestation Day 15 at doses of approximately 3 to 12 mg/m2 (at doses less than the recommended human daily dose on a mg/m2 basis).
In rats, azacitidine was clearly embryotoxic when given an intraperitoneal injection on gestation Days 4 to 8 (postimplantation) at a dose of 6 mg/m2 (at doses less than the recommended human daily dose on a mg/m2 basis), although treatment in the preimplantation period (on gestation Days 1 to 3) had no adverse effect on the embryos. Azacitidine caused multiple fetal abnormalities in rats after a single intraperitoneal dose of 3 to 12 mg/m2 (at doses less than the recommended human daily dose on a mg/m2 basis) given on gestation Days 9, 10, 11, or 12. In this study, azacitidine caused fetal death when administered at 3 to 12 mg/m2 on gestation Days 9 and 10; average live animals per litter was reduced to 9% of control at the highest dose on gestation Day 9. Fetal anomalies included: CNS anomalies (exencephaly/encephalocele), limb anomalies (micromelia, club foot, syndactyly, oligodactyly), and others (micrognathia, gastroschisis, edema, and rib abnormalities).
8.2 Lactation
Risk Summary
There are no data regarding the presence of azacitidine in human milk or the effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with ONUREG and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
ONUREG can cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential before starting ONUREG.
Contraception
Infertility
Based on animal data, ONUREG may impair male or female fertility [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of ONUREG in pediatric patients have not been established.
8.5 Geriatric Use
Of the 238 patients in QUAZAR who received ONUREG, 72% were 65 years of age or older, while 12% were 75 years of age or older. No overall differences in safety or effectiveness of ONUREG were observed between these patients and younger patients.
8.6 Renal Impairment
Monitor patients with severe renal impairment (creatinine clearance [CLcr] 15 to 29 mL/min calculated by Cockcroft-Gault formula) more frequently for adverse reactions and modify the ONUREG dosage for adverse reactions [see Dosage and Administration (2.3)].
No dose adjustment of ONUREG is recommended for patients with mild to severe renal impairment (CLcr 15 to 89 mL/min) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
ONUREG has not been studied in patients with pre-existing severe hepatic impairment (total bilirubin > 3 × ULN).
A recommended dosage of ONUREG has not been established for patients with moderate hepatic impairment (total bilirubin > 1.5 to 3 × ULN).
No dose adjustment of ONUREG is recommended for patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1 to 1.5 × ULN and any AST) [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
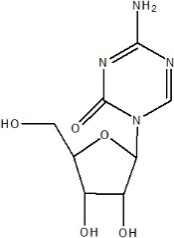
Azacitidine is a nucleoside metabolic inhibitor with a molecular formula of C8H12N4O5 and a molecular weight of 244 g/mol. The chemical name is: 4-amino-1-β-D-ribofuranosyl-s-triazin-2(1H)-one and the chemical structural is:
Azacitidine is a white to off-white solid. Azacitidine was found to be soluble in aqueous media across a pH range from 1.0 to 7.0.
ONUREG (azacitidine) is supplied as film-coated tablets containing 200 mg or 300 mg of azacitidine for oral use. Each core tablet contains the following inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, and silicified microcrystalline cellulose. The 200 and 300 mg tablet coating contains hypromellose, lactose monohydrate, polyethylene glycol, titanium dioxide, and triacetin. In addition, the 200 mg tablet coating contains iron oxide red and the 300 mg tablet coating contains black iron oxide, iron oxide red, and iron oxide yellow.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Azacitidine is a pyrimidine nucleoside analog of cytidine that inhibits DNA/RNA methyltransferases. Azacitidine is incorporated into DNA and RNA following cellular uptake and enzymatic biotransformation to nucleotide triphosphates.
Incorporation of azacitidine into the DNA of cancer cells in vitro, including acute myeloid leukemia cells, inhibited DNA methyltransferases, reduced DNA methylation and altered gene expression, including re-expression of genes regulating tumor suppression and cell differentiation. Incorporation of azacitidine into the RNA of cancer cells, including leukemic cells, inhibited RNA methyltransferases, reduced RNA methylation, decreased RNA stability and decreased protein synthesis.
Antileukemic activity of azacitidine was demonstrated by reduction of cell viability and induction of apoptosis in AML cell lines in vitro. Azacitidine decreased tumor burden and increased survival in leukemic tumor models in vivo.
12.2 Pharmacodynamics
Greater reduction in global DNA methylation was observed with higher azacitidine plasma exposure in patients with AML administered ONUREG for 14 days of a 28-day cycle.
12.3 Pharmacokinetics
The systemic exposure of azacitidine is approximately dose proportional over the dose range of 120 mg to 600 mg once daily of ONUREG (0.4 to 2 times the recommended dosage). Following a single 300 mg dose of ONUREG, the mean (coefficient of variation [CV%]) Cmax of azacitidine was 145 ng/mL (64%) and the mean AUC of azacitidine was 242 ng h/mL (65%). No accumulation was observed following ONUREG 300 mg once daily.
Absorption
The mean oral bioavailability is approximately 11% relative to subcutaneous administration. The median time to peak plasma concentration of azacitidine is 1 hour.
Distribution
The mean (CV%) apparent volume of distribution (Vz/F) of azacitidine is 881 L (67%). The in vitro serum protein binding of azacitidine is approximately 6% to 12%. The blood-to-plasma ratio is approximately 0.3.
Elimination
The mean (CV%) terminal half-life is approximately 0.5 hours (27%) and the apparent clearance (CL/F) is 1240 L/hour (64%).
Specific Populations
Age (46 years to 93 years), sex, body weight (39.3 kg to 129 kg), mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1 to 1.5 × ULN and any AST), and mild to moderate renal impairment (CLcr 30 to 89 mL/min) have no clinically meaningful effect on the pharmacokinetics of oral azacitidine. The effects of race/ethnicity, moderate to severe hepatic impairment (total bilirubin > 1.5 × ULN and any AST), and severe renal impairment (CLcr 15 to 29 mL/min) on the pharmacokinetics of oral azacitidine is unknown.
Severe renal impairment increased azacitidine exposure by approximately 70% after a single or 41% after multiple subcutaneous daily administration.
Drug Interaction Studies
Effect of Gastric Acid Reducing Agents on Azacitidine:
Coadministration of omeprazole (a proton pump inhibitor) with ONUREG increased azacitidine AUC0-INF by 19% and had no effect on Cmax.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Azacitidine does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, or CYP2E1 at clinically relevant concentrations. Azacitidine is not an inducer of CYP1A2, CYP2C19, or CYP3A.
Transporter Systems: Azacitidine is not a substrate of P-glycoprotein (P-gp). Azacitidine does not inhibit P-gp, breast cancer resistance protein (BCRP), organic anion transporters (OAT) OAT1 and OAT3, organic anion transporting polypeptides (OATP) OATP1B1 and OATP1B3, or organic cation transporter (OCT) OCT2 at clinically relevant concentrations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The potential carcinogenicity of azacitidine was evaluated in mice and rats. Azacitidine induced tumors of the hematopoietic system in female mice at 2.2 mg/kg (6.6 mg/m2, approximately 4% of the recommended human daily dose of oral azacitidine on a mg/m2 basis) administered intraperitoneal 3 times per week for 52 weeks. An increased incidence of tumors in the lymphoreticular system, lung, mammary gland, and skin was seen in mice treated with intraperitoneal azacitidine at 2 mg/kg (6 mg/m2, approximately 3% of the recommended human daily dose of oral azacitidine on a mg/m2 basis) once a week for 50 weeks. A tumorigenicity study in rats dosed twice weekly at 15 or 60 mg/m2 (approximately 8% to 32% of the recommended human daily dose of oral azacitidine on a mg/m2 basis) revealed an increased incidence of testicular tumors compared with controls.
The mutagenic and clastogenic potential of azacitidine was tested in in vitro bacterial systems Salmonella typhimurium strains TA100 and several strains of trpE8, Escherichia coli strains WP14 Pro, WP3103P, WP3104P, and CC103; in an in vitro forward gene mutation assay in mouse lymphoma cells and human lymphoblast cells; and in an in vitro micronucleus assay in mouse L5178Y lymphoma cells and Syrian hamster embryo cells. Azacitidine was mutagenic in bacterial and mammalian cell systems. The clastogenic effect of azacitidine was shown by the induction of micronuclei in L5178Y mouse cells and Syrian hamster embryo cells.
Administration of azacitidine by intraperitoneal injection to male mice at 9.9 mg/m2 (at doses less than the recommended human daily dose on a mg/m2 basis) daily for 3 days prior to mating with untreated female mice resulted in decreased fertility and loss of offspring during subsequent embryonic and postnatal development. Treatment of male rats 3 times per week for 11 or 16 weeks at doses of 15 to 30 mg/m2 (at doses less than the recommended human daily dose on a mg/m2 basis) resulted in decreased weight of the testes and epididymides, decreased sperm counts accompanied by decreased pregnancy rates, and increased loss of embryos in mated females. In a related study, male rats treated for 16 weeks at 24 mg/m2 resulted in an increase in abnormal embryos in mated females when examined on Day 2 of gestation.
-
14 CLINICAL STUDIES
The efficacy of ONUREG was evaluated in QUAZAR (NCT01757535), a multicenter, randomized, double-blind, placebo-controlled study. Eligible patients were ages 55 years or older, had AML, and were within 4 months of achieving first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) with intensive induction chemotherapy. Patients may have received consolidation (see Table 4). Patients were excluded if they were candidates for hematopoietic stem cell transplantation at the time of screening.
A total of 472 patients who completed induction with or without consolidation therapy were randomized 1:1 to receive ONUREG 300 mg (n=238) or placebo (n=234) orally on Days 1 through 14 of each 28-day cycle. Randomization was stratified by age at time of induction therapy (55 to 64 vs. ≥ 65 years), cytogenetic risk category at time of induction therapy (intermediate risk vs. poor risk), prior history of MDS/CMML (yes vs. no), and received consolidation therapy following induction therapy (yes vs. no). Baseline demographic and disease characteristics are shown in Table 4.
Table 4: Baseline Demographics and Disease-Related Characteristics in QUAZAR Parameter ONUREG
(N=238)Placebo
(N=234)AML=Acute Myeloid Leukemia, MDS=Myelodysplastic Syndrome, CMML=Chronic Myelomonocytic Leukemia, ECOG=Eastern Cooperative Oncology Group, CR=Morphologic Complete Remission, CRi=Morphologic complete remission with incomplete blood count recovery.
Source for Intermediate and Poor Risk: National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Acute Myeloid Leukemia. National Comprehensive Cancer Network website. Available at http://www.nccn.org/professionals/physician_gls/PDF/aml.pdf. Accessed 01 Mar 2011.
1 Intermediate risk was defined as normal cytogenetics +8, t(9;11), or Other undefined.
2 Poor risk was defined as Complex (≥ 3 abnormalities): -5; 5q-; -7; 7q-; 11q23 - non t(9;11); inv(3); t(3;3); t(6;9); or t(9;22).Age (years)
Median (Min, Max)
68.0 (55, 86)
68.0 (55, 82)
Age Category, n (%)
< 65 years
66 (28)
68 (29)
65 years to < 75 years
144 (61)
142 (61)
≥ 75 years
28 (12)
24 (10)
Sex, n (%)
Male
118 (50)
127 (54)
Female
120 (50)
107 (46)
Race, n (%)
White
216 (91)
197 (84)
Black or African American
2 (1)
6 (3)
Asian
6 (3)
20 (9)
Other
12 (5)
11 (5)
Not Collected or Reported
2 (1)
0 (0)
ECOG Performance Status, n (%)
0
116 (49)
111 (47)
1
101 (42)
106 (45)
2
21 (9)
15 (6)
3
0 (0)
2 (1)
Cytogenetic Risk Status at Diagnosis, n (%)
Intermediate Risk1
203 (85)
203 (87)
Poor Risk2
35 (15)
31 (13)
Initial AML Classification, n (%)
AML with recurrent genetic abnormalities
39 (16)
46 (20)
AML with myelodysplasia-related changes
49 (21)
42 (18)
Therapy related myeloid neoplasms
2 (1)
0 (0)
AML not otherwise specified
148 (62)
145 (62)
Missing
0 (0)
1 (< 1)
Type of AML, n (%)
Primary (de novo)
213 (89)
216 (92)
Secondary
25 (11)
18 (8)
Induction Response
CR
187 (79)
197 (84)
CRi
51 (21)
37 (16)
Consolidation following induction therapy
None
52 (22)
42 (18)
1 cycle
110 (46)
102 (44)
2 cycles
70 (29)
77 (33)
3 cycles
6 (3)
13 (6)
Disease status at study baseline
CR
185 (78)
181 (77)
CRi
44 (18)
38 (16)
Not in CR or CRi
9 (4)
13 (6)
Not Reported
0
2 (1)
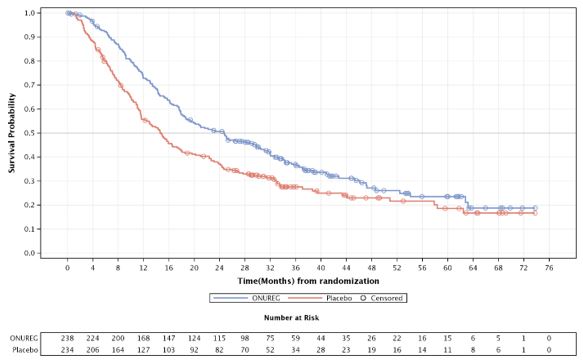
The efficacy of ONUREG was established on the basis of overall survival (OS). The trial demonstrated a statistically significant improvement in OS for patients randomized to ONUREG compared to placebo. A subgroup analysis showed consistency in the OS benefit for patients in either CR or CRi. The efficacy results are summarized in Table 5 and Figure 1.
Table 5: Efficacy Results (ITT Population) in QUAZAR ONUREG
(N=238)Placebo
(N=234)1 The hazard ratio is from a Cox proportional hazards model stratified by age (55 to 64 vs. ≥65 years), cytogenetic risk category at time of induction therapy (intermediate risk vs. poor risk), and received consolidation therapy (yes vs. no). The p-value is two-sided from a log-rank test stratified by the same factors. Overall Survival
OS Events, n (%)
158 (66)
171 (73)
Median OS (95% CI) Months
24.7 (18.7, 30.5)
14.8 (11.7, 17.6)
Hazard Ratio (95% CI)1
0.69 (0.55, 0.86)
p value1
0.0009
Figure 1: Kaplan-Meier Curve for Overall Survival (ITT Population) in QUAZAR
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ONUREG tablets are available as:
- 200 mg: pink, oval, film-coated tablets with debossed "200" on one side and "ONU" on the other side.
- 300 mg: brown, oval, film-coated tablets with debossed "300" on one side and "ONU" on the other side.
Table 6 lists the package configurations and strengths.
Table 6: ONUREG Package Configurations and NDC Numbers Package Configuration Tablet Strength NDC Number One blister card containing 7 tablets
200 mg
59572-730-07
One blister card containing 7 tablets
300 mg
59572-740-07
Storage
-
Store bottles at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Keep bottle tightly closed.
Store and dispense in the original bottle (with two desiccant canisters). -
Store blisters at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Store in the original aluminum-aluminum blisters.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myelosuppression
Advise patients of the risk of myelosuppression with ONUREG and of the need to monitor complete blood counts before and during treatment [see Warnings and Precautions (5.2)].
Gastrointestinal Toxicity
Advise patients of the risk of gastrointestinal toxicity with ONUREG and of the potential need to use anti-emetic or anti-diarrheal medications during treatment [see Adverse Reactions (6.1)].
Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with ONUREG and for at least 6 months after the last dose [see Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with ONUREG and for at least 3 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with ONUREG and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Administration
Advise patients to take ONUREG with or without food at about the same time each day and how to make up a missed or vomited dose. Advise patients to swallow tablets whole. Advise patients not to cut, crush, or chew the tablets [see Dosage and Administration (2.2)].
Storage Instructions
Advise patients to keep ONUREG in the original container (bottles or blisters). If bottles are dispensed, advise patients to keep the container tightly closed with both desiccant canisters inside and to not eat the desiccant canisters [see How Supplied/Storage and Handling (16)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: October 2022Patient Information
ONUREG® (on-u-reg)
(azacitidine) tablets, for oral useWhat is ONUREG?
ONUREG is a prescription medicine used for continued treatment of adults with acute myeloid leukemia (AML) who:
- had a first complete remission (CR) following intensive induction chemotherapy with or without recovery of your blood cell counts, and
- who are not able to complete intensive curative therapy.
It is not known if ONUREG is safe and effective in children under 18 years of age.
Do not take ONUREG if you:
- are allergic to azacitidine or any of the ingredients in ONUREG. See the end of this leaflet for a complete list of ingredients in ONUREG.
Before taking ONUREG, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney or liver problems.
-
are pregnant or plan to become pregnant. ONUREG can harm your unborn baby.
Females who are able to become pregnant:- o Your healthcare provider should perform a pregnancy test before you start treatment with ONUREG.
- o You should use effective birth control (contraception) during treatment and for at least 6 months after your last dose of ONUREG.
- o Tell your healthcare provider right away if you become pregnant during treatment with ONUREG.
-
Males with a female sexual partner who can become pregnant:
- o You should use effective birth control (contraception) during treatment and for at least 3 months after your last dose of ONUREG.
- are breastfeeding or plan to breastfeed. It is not known if ONUREG passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose of ONUREG.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take ONUREG?
- Take ONUREG exactly as your healthcare provider tells you to take it.
-
Your healthcare provider will prescribe an anti-nausea medicine for you to take to help prevent nausea and vomiting during your treatment with ONUREG.
- o Take the anti-nausea medicine 30 minutes before each dose of ONUREG.
- o Your healthcare provider may decide to stop the anti-nausea medicine after your second cycle of ONUREG, if you do not have any nausea or vomiting.
- Take ONUREG by mouth 1 time each day beginning on Day 1 through Day 14 of each 28-day cycle.
- Take ONUREG with or without food at about the same time each day.
- Swallow ONUREG tablets whole. Do not cut, crush, or chew the tablets.
- If the powder from ONUREG tablets comes in contact with your skin, wash the area well right away with soap and water.
- If the powder from ONUREG tablets comes in contact with your eyes or mouth (mucous membranes), flush the area right away with water.
- If you miss a dose of ONUREG, or if you do not take your dose at the usual time, take the dose as soon as possible that day. Take your next dose at the regular time the next day. Do not take 2 doses on the same day to make up for a missed dose.
- If you vomit after taking a dose of ONUREG, do not take another dose on the same day. Take your next dose at the regular time the next day.
What are the possible side effects of ONUREG?
ONUREG can cause serious side effects, including:
-
New or worsening low white blood cell counts (neutropenia). New or worsening low white blood cell counts are common but can also be severe during treatment with ONUREG. If your white blood cell counts become very low, you are at increased risk for infections. Your healthcare provider will check your white blood cell counts before and during treatment with ONUREG. Your healthcare provider may prescribe a medicine to help increase your white blood cell count if needed.
Tell your healthcare provider right away if you get any of the following symptoms:
- o fever or chills
- o body aches
- o feeling very tired or weak
- o unusual headaches
- New or worsening low platelet counts (thrombocytopenia). Low platelet counts are common but can also be severe during treatment with ONUREG. Your healthcare provider will check your platelet counts before and during treatment with ONUREG. Tell your healthcare provider right away if you have any unusual bruising or bleeding. Your healthcare provider may change your dose or tell you to stop taking ONUREG if you have low blood cell counts.
ONUREG may cause fertility problems in males and females, which may affect your ability to have children. Talk with your healthcare provider if you have concerns about fertility.
The most common side effects of ONUREG include:
- nausea and vomiting. See "How should I take ONUREG?"
- diarrhea. You may need to be treated with anti-diarrheal medicines.
- tiredness or weakness
- constipation
- stomach area (abdominal) pain
- pneumonia
- joint pain
- decreased appetite
- pain in arms or legs
- dizziness
These are not all of the possible side effects of ONUREG. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ONUREG?
- Store bottles or blisters of ONUREG tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store ONUREG tablets in the original bottle or the original aluminum-aluminum blisters.
- Bottles of ONUREG contain 2 drying agent (desiccant) canisters. Do not eat the desiccant canisters.
- Keep the ONUREG bottle tightly closed.
- Talk to your healthcare provider about how to safely throw away (dispose of) any unused or expired ONUREG.
Keep ONUREG and all medicines out of the reach of children
General information about the safe and effective use of ONUREG.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ONUREG for a condition for which it was not prescribed. Do not give ONUREG to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ONUREG that is written for health professionals.
What are the ingredients in ONUREG?
Active ingredient: azacitidine
Inactive ingredients:
Each core tablet contains: croscarmellose sodium, magnesium stearate, mannitol, and silicified microcrystalline cellulose.
The pink 200 mg tablet coating contains: hypromellose, iron oxide red, lactose monohydrate, polyethylene glycol, titanium dioxide, and triacetin.
The brown 300 mg tablet coating contains: black iron oxide, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, polyethylene glycol, titanium dioxide, and triacetin.
Marketed by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
ONUREG® is a trademark of Celgene Corporation, a Bristol-Myers Squibb company.
ONUPPI V4 10/2022
For more information, go to www.ONUREG.com or call 1-800-721-5072. -
PRINCIPAL DISPLAY PANEL - 200 mg Bottle Label
NDC: 59572-730-14
ONUREG™
(azacitidine) tablets200 mg
Swallow tablets whole.
Do not cut, crush, or chew the tablets.Rx only
14 TabletsCAUTION: Hazardous Agent
-
PRINCIPAL DISPLAY PANEL - 200 mg Blister Pack Carton
NDC: 59572-730-07
Rx onlyONUREG™
(azacitidine) tablets200 mg
Each tablet contains 200 mg of azacitidine.
Swallow tablets whole. Do not cut, crush, or chew the tablets.
One Blister Card
Containing 7 TabletsBristol Myers Squibb
CAUTION: Hazardous Agent
-
PRINCIPAL DISPLAY PANEL - 300 mg Blister Pack Carton
NDC: 59572-740-07
Rx onlyONUREG™
(azacitidine) tablets300 mg
Each tablet contains 200 mg of azacitidine.
Swallow tablets whole. Do not cut, crush, or chew the tablets.
One Blister Card
Containing 7 TabletsBristol Myers Squibb
CAUTION: Hazardous Agent
-
INGREDIENTS AND APPEARANCE
ONUREG
azacitidine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59572-730 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZACITIDINE (UNII: M801H13NRU) (AZACITIDINE - UNII:M801H13NRU) AZACITIDINE 200 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color PINK Score no score Shape OVAL Size 19mm Flavor Imprint Code ONU;200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59572-730-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 2 NDC: 59572-730-07 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 12/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214120 09/01/2020 ONUREG
azacitidine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59572-740 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZACITIDINE (UNII: M801H13NRU) (AZACITIDINE - UNII:M801H13NRU) AZACITIDINE 300 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color BROWN Score no score Shape OVAL Size 17mm Flavor Imprint Code ONU;300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59572-740-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 2 NDC: 59572-740-07 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 12/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214120 09/01/2020 Labeler - Celgene Corporation (174201137)
Trademark Results [ONUREG]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONUREG 90142491 not registered Live/Pending |
Celgene Corporation 2020-08-27 |
 ONUREG 88490065 not registered Live/Pending |
Celgene Corporation 2019-06-26 |
 ONUREG 88131315 not registered Live/Pending |
Celgene Corporation 2018-09-25 |
 ONUREG 86115220 4559839 Live/Registered |
Celgene Corporation 2013-11-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.