REBINYN ((coagulation factor ix- recombinant, glycopegylated kit
REBINYN by
Drug Labeling and Warnings
REBINYN by is a Prescription medication manufactured, distributed, or labeled by Novo Nordisk, Novo Nordisk A/S - Hax, Novo Nordisk A/S - 25a-B. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REBINYN safely and effectively. See full prescribing information for REBINYN.
REBINYN® (Coagulation Factor IX (Recombinant), GlycoPEGylated)
Lyophilized Powder for solution for intravenous injection
Initial U.S. Approval: 2017INDICATIONS AND USAGE
REBINYN, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA-derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
Limitations of Use: REBINYN is not indicated for routine prophylaxis in the treatment of patients with hemophilia B. REBINYN is not indicated for immune tolerance induction in patients with hemophilia B (1).
DOSAGE AND ADMINISTRATION
For intravenous infusion after reconstitution only (2).
- Each carton and vial label for REBINYN states the actual Factor IX potency in international units (IU) (2.1).
- Recommended dose for on-demand treatment and control of bleeding episodes: 40 IU/kg body weight for minor and moderate bleeds, and 80 IU/kg body weight for major bleeds. Additional doses of 40 IU/kg can be given (2.1).
- Recommended dose for perioperative management: Pre-operative dose of 40 IU/kg body weight for minor surgery, and 80 IU/kg body weight for major surgery. As clinically needed for the perioperative management of bleeding, repeated doses of 40 IU/kg (in 1-3 day intervals) within the first week after major surgery may be administered. Frequency may be extended to once weekly after the first week until bleeding stops and healing is achieved (2.1).
DOSAGE FORMS AND STRENGTHS
REBINYN is available as a lyophilized powder in single-use vials of 500, 1000, and 2000 IU (3).
CONTRAINDICATIONS
Do not use in patients who have known hypersensitivity to REBINYN or its components, including hamster proteins (4).
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions, including anaphylaxis, may occur. Should hypersensitivity reactions occur, discontinue REBINYN and administer appropriate treatment (5.1).
- Development of neutralizing antibodies (inhibitors) may occur. Perform an assay that measures Factor IX inhibitor concentration if bleeding is not controlled with the recommended dose of REBINYN or if the expected plasma Factor IX activity levels are not attained (5.2, 5.5).
- The use of Factor IX-containing products has been associated with the development of thrombotic complications (5.3).
- Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used (5.5).
ADVERSE REACTIONS
The most frequently reported adverse reactions (≥ 1%) were itching and injection site reactions (6).
Animals administered repeat doses of REBINYN showed accumulation of PEG in the choroid plexus. The potential clinical implications of these animal findings are unknown (6.3).
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-877-668-6777 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pediatric Use: No dose adjustment is needed (8.4).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Guidelines
2.2 Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Inhibitors
5.3 Thrombotic Events
5.4 Nephrotic Syndrome
5.5 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Neurologic Considerations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
REBINYN, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA-derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
Limitations of Use: REBINYN is not indicated for routine prophylaxis in the treatment of patients with hemophilia B. REBINYN is not indicated for immune tolerance induction in patients with hemophilia B.
-
2 DOSAGE AND ADMINISTRATION
For intravenous infusion after reconstitution only.
2.1 Dosing Guidelines
- Dose and duration of treatment depend on the location and extent of bleeding, and the patient’s clinical condition.
- If monitoring of Factor IX activity is performed, use a chromogenic assay or selected one-stage clotting assay validated for use with REBINYN [see Warnings and Precautions (5.5)].
- Each carton and vial label for REBINYN states the actual Factor IX potency in IU.
On-demand Treatment and Control of Bleeding Episodes
REBINYN dosing for on-demand treatment and control of bleeding episodes is provided in Table 1.
Table 1: Dosing for On-demand Treatment and Control of Bleeding Episodes
Type of bleeding
Recommended dose
IU/kg body weight
Additional information
Minor and moderate
For example: Uncomplicated joint bleeds, minor muscular bleeds, mucosal or subcutaneous bleeds
40
A single dose should be sufficient for minor and moderate bleeds. Additional doses of 40 IU/kg can be given.
Major
For example: Intracranial, retroperitoneal, iliopsoas and neck bleeds, muscle bleeds with compartment syndrome and bleeds associated with a significant decrease in the hemoglobin level
80
Additional doses of 40 IU/kg can be given.
Perioperative Management
REBINYN dosing for perioperative management is provided in Table 2.
Table 2: Dosing for Perioperative Management
Type of surgical procedure
Recommended dose
IU/kg body weight
Additional Information
Minor
For example: Implanting pumps in subcutaneous tissue, skin biopsies or simple dental procedures
40
A single pre-operative dose should be sufficient. Additional doses can be given if needed.
Major
For example: Body cavity is entered, mesenchymal barrier is crossed, fascial plane is opened, organ is removed, normal anatomy is operatively altered
80
Pre-operative dose
40
As clinically needed for the perioperative management of bleeding, repeated doses of 40 IU/kg (in 1-3 day intervals) within the first week after major surgery may be administered.*
Due to the long half-life of REBINYN, the frequency of dosing in the post-surgical setting may be extended to once weekly after the first week until bleeding stops and healing is achieved.
*See 12.3 Pharmacokinetics, Table 8
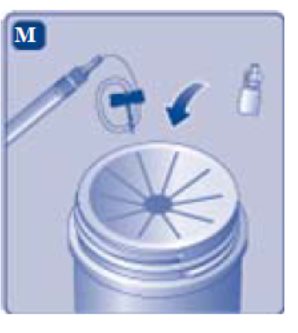
2.2 Reconstitution
- Always wash hands and ensure that the area is clean before performing the reconstitution procedures.
- Use aseptic technique during the reconstitution procedures.
- If the patient uses more than one vial of REBINYNper infusion, reconstitute each vial according to the following instructions.
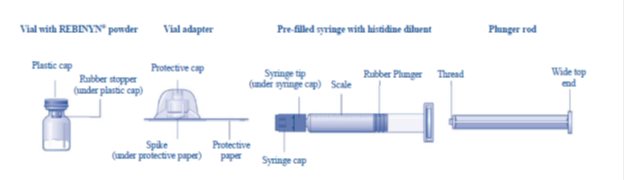
Overview of REBINYN Package
The instructions below serve as a general guideline for reconstitution of REBINYN. For full instructions, refer to the FDA-approved patient information and Instructions for Use.
Reconstitution
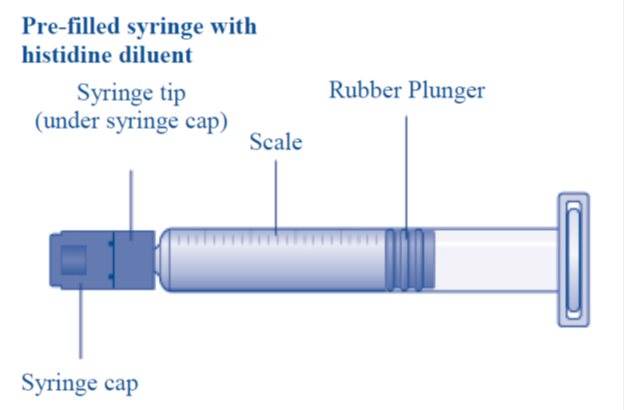
- 1. Bring the REBINYNvial and the pre-filled diluent syringe to room temperature.
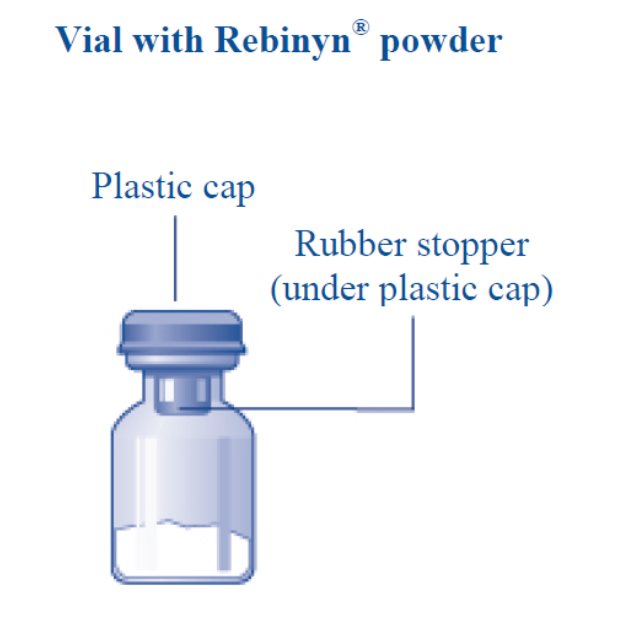
- 2. Remove the plastic cap from the REBINYNvial.
- 3. Wipe the rubber stopperon the vial with a sterile alcohol swab and allow it to dry prior to use.
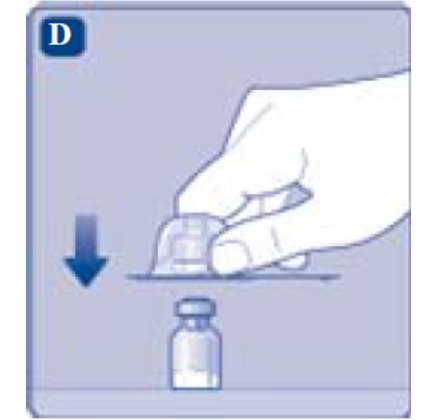
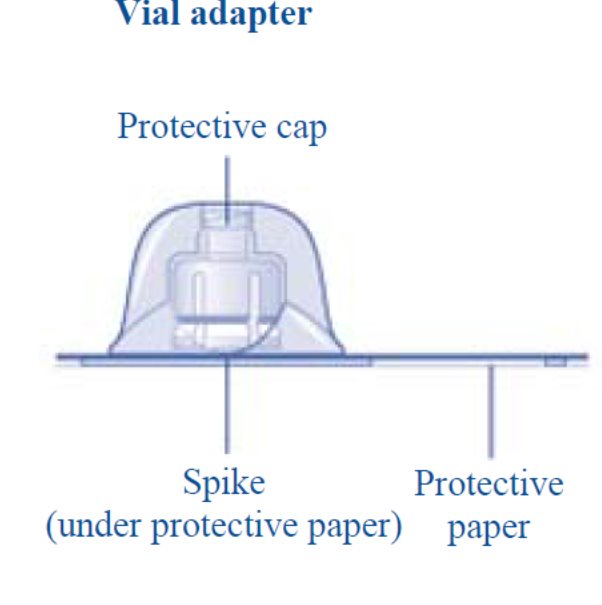
- 4. Remove the protective paper from the vial adapter. Do not remove the vial adapter from the protective cap.
- 5. Place the vial on a flat and solid surface. While holding the protective cap, place the vial adapter over the REBINYN vial and press down firmly on the protective cap until the vial adapter spike penetrates the rubber stopper.
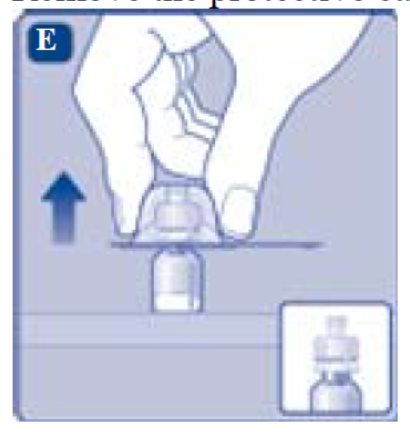
- 6. Remove the protective cap from the vial adapter.
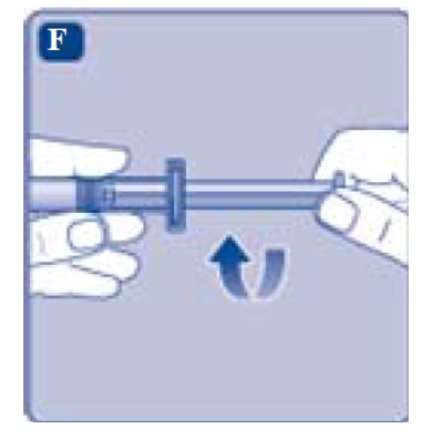
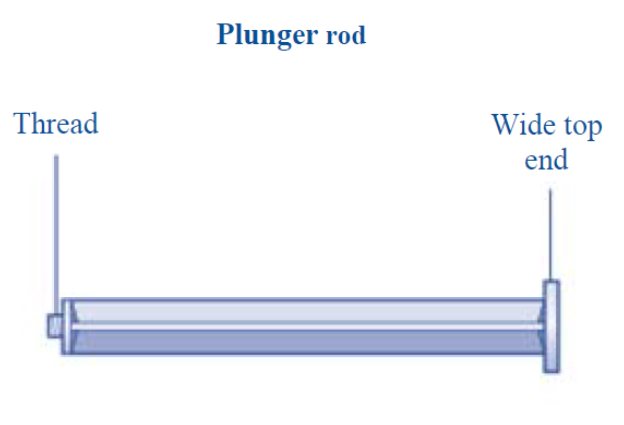
- 7. Grasp the plunger rod as shown in the diagram. Attach the plunger rod to the syringe by holding the plunger rod by the wide top end. Turn the plunger rod clockwise into the rubber plunger inside the pre-filled diluent syringe until resistance is felt.
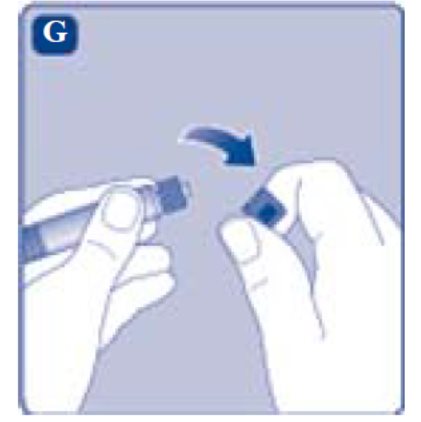
- 8. Break off the syringe cap from the pre-filled diluent syringe by snapping the perforation of the cap.
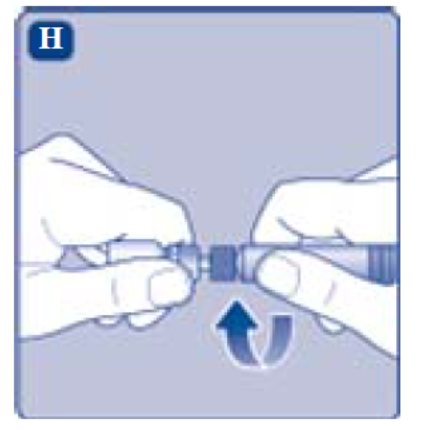
- 9. Connect the pre-filled diluent syringe to the vial adapter by turning it clockwise until it is secured.
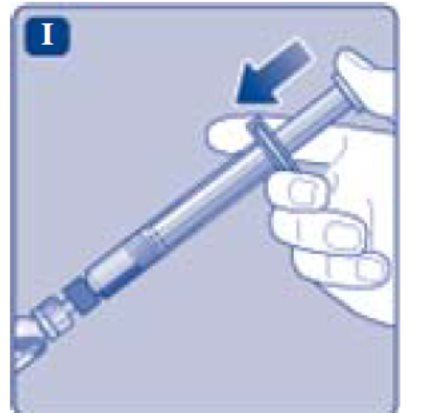
- 10. Push the plunger rod to slowly inject all the diluent into the vial.
- 11. Without removing the syringe, gently swirl the REBINYN vial until all of the powder is dissolved.
- 12. Administer the REBINYN solution immediately [see Administration (2.3)]. If not used immediately after reconstitution, store the solution in the vial with the vial adapter and the syringe attached, at room temperature ≤ 86°F (30°C). Do not store for longer than 4 hours.
2.3 Administration
For intravenous infusion only.
- Accidental needle stickwith a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. If a needle stick occurs, obtain immediate medical attention. Place needles in a sharps container after single use.
- Inspect the reconstituted REBINYNsolution visually prior to administration [see Description (11)]. The solution should be clear and have no particles. Do not use if particulate matter or discoloration is observed.
- Do not administer REBINYNin the same tubing or container with other medicinal products.
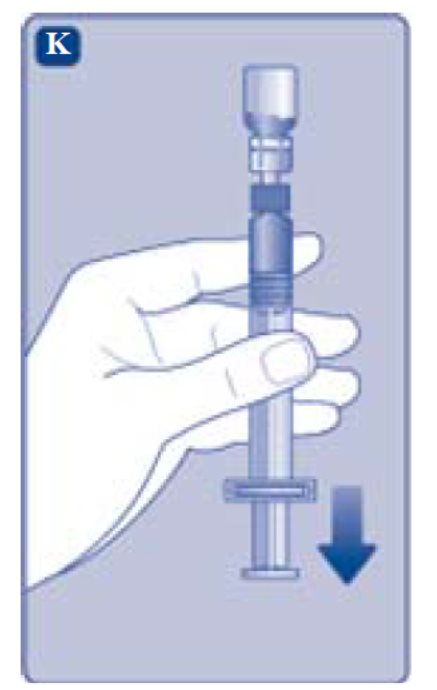
- 1. Invert the REBINYNvial and slowly draw the solution into the syringe.
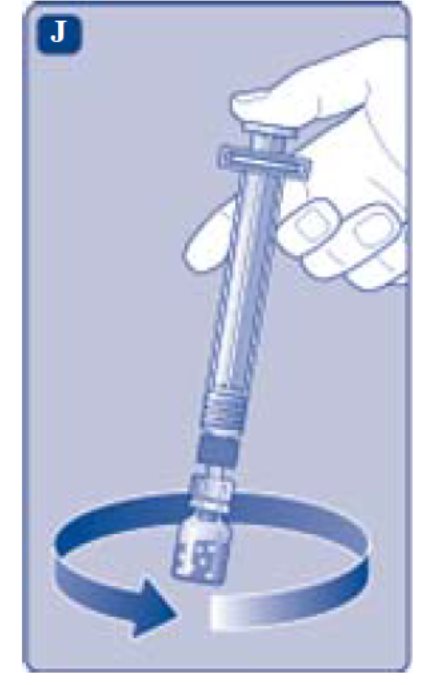
- 2. Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- 3. Attach the syringe to the luer end of an infusion needle set.
- 4. Infuse the reconstituted REBINYNintravenously slowly over 1 to 4 minutes.
- 5. After infusion, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused REBINYNand other waste materials.
Caution: The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer REBINYN through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
If you encounter any problems with attaching the pre-filled histidine-diluent syringe to any Luer‐lock compatible device, please contact Novo Nordisk at (844) 303-4448.
-
3 DOSAGE FORMS AND STRENGTHS
REBINYN is available as a white to off-white lyophilized powder in single-use vials containing nominally 500, 1000, or 2000 IU per vial. Each carton and vial label for REBINYN states the actual Factor IX potency in IU.
After reconstitution with 4 mL of histidine diluent, the reconstituted solution contains approximately 125, 250 or 500 IU per mL of REBINYN respectively.
-
4 CONTRAINDICATIONS
REBINYN is contraindicated in patients who have known hypersensitivity to REBINYN or its components (including hamster proteins) [see Warnings and Precautions (5.1) and Description (11)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, are possible with REBINYN. The product may contain traces of hamster proteins which in some patients may cause allergic reactions. Early signs of allergic reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, difficulty breathing, wheezing, urticaria, and itching. Observe patients for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of exposure to the product. Discontinue use of REBINYN if allergic- or anaphylactic - type reactions occur, and initiate appropriate treatment.
5.2 Inhibitors
The formation of inhibitors (neutralizing antibodies) to Factor IX may occur during Factor replacement therapy in the treatment of hemophilia B. Monitor all patients using clinical observations and laboratory tests for the development of inhibitors [see Warnings and Precautions (5.5)].
An association between the development of Factor IX inhibitors and allergic reactions has been reported. Evaluate patients experiencing allergic reactions for the presence of an inhibitor. Patients with Factor IX inhibitors may be at an increased risk of severe allergic reactions with subsequent exposure to Factor IX.
5.3 Thrombotic Events
The use of Factor IX-containing products has been associated with thrombotic complications. Due to the potential risk of thrombotic complications, monitor patients for early signs of thrombotic and consumptive coagulopathy when administering this product to patients with liver disease, post-operatively, to newborn infants, or to patients at risk of thrombosis or disseminated intravascular coagulation (DIC). In each of these situations, the benefit of treatment with REBINYN should be weighed against the risk of these complications.
5.4 Nephrotic Syndrome
Nephrotic syndrome has been reported following immune tolerance induction therapy with Factor IX products in hemophilia B patients with Factor IX inhibitors, often with a history of allergic reactions to Factor IX. The safety and efficacy of using REBINYN for immune tolerance induction have not been established.
5.5 Monitoring Laboratory Tests
If monitoring of Factor IX activity is performed, use a chromogenic assay or selected one-stage clotting assay validated for use with REBINYN [see Dosage and Administration (2)].
The one-stage clotting assay results can be significantly affected by the type of activated partial thromboplastin time (aPTT) reagent used, which can result in over- or under-estimation of Factor IX activity. Avoid the use of silica-based reagents, as some may overestimate the activity of REBINYN. If a validated one-stage clotting or chromogenic assay is not available locally, then use of a reference laboratory is recommended.
If bleeding is not controlled with the recommended dose of REBINYN, or if the expected Factor IX activity levels in plasma are not attained, then perform a Bethesda assay to determine if Factor IX inhibitors are present.
-
6 ADVERSE REACTIONS
Common adverse reactions (incidence ≥ 1%) reported in clinical trials for REBINYN were itching and injection site reactions.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
During the clinical development program, 115 previously treated male patients received at least one dose of REBINYN [seeClinical Studies (14)]. A previously treated patient was defined as a subject with a history of at least 150 exposure days to other Factor IX products (adolescent/adult subjects) or 50 exposure days to other Factor IX products (pediatric subjects), and no history of inhibitors. There were a total of 8801 exposure days, equivalent to 170 patient-years. A total of 40 patients (35%) were treated for more than 2 years.
Adverse reactions are shown in Table 3.
- Table 3: Summary of Adverse Reactions in Previously Treated Patients
System Organ Class
Adverse Reaction
Number of subjects (%)
General disorders and administration site conditions
Injection site reactions
4 (4)
Immune system disorders
Hypersensitivity
1 (1)
Skin and subcutaneous tissue disorders
Itching
3 (3)
6.2 Immunogenicity
Subjects were monitored for inhibitory antibodies to factor IX prior to dosing, on a monthly basis for the first three months, every two months up to one year, every three months for an additional year, and then every 6 months until end of trial.
No inhibitors were reported in the clinical trials in previously treated patients.
In an ongoing trial in previously untreated patients, anaphylaxis has occurred with development of a factor IX inhibitor following treatment with REBINYN. Inhibitor development and anaphylactic reactions are more likely to occur during the early phases of factor IX replacement therapy [seeWarnings and Precautions (5.1, 5.2)].
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
6.3 Neurologic Considerations
Animals administered repeat doses of REBINYN showed accumulation of PEG in the choroid plexus [see Animal Toxicology and/or Pharmacology (13.2)]. The potential clinical implications of these animal findings are unknown. The physician should consider whether the patient may be vulnerable, such as infants and children who have developing brains and patients who are cognitively impaired. Physician’s discretion is advised with regard to neurocognitive assessments, taking into consideration factors such as duration of use, cumulative dose, age of the patient and related comorbidities that are likely to increase the risks to patients. Adverse neurologic reactions should be reported.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with REBINYN use in pregnant women to determine whether there is a drug-associated risk. Animal reproduction studies have not been conducted with REBINYN. It is unknown whether REBINYN can cause fetal harm when administered to a pregnant woman or can affect fertility. REBINYN should be given to a pregnant woman only if clearly needed. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of REBINYN in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for REBINYN and any potential adverse effects on the breastfed infant from REBINYN or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy of REBINYN were evaluated in 43 previously treated pediatric patients [seeClinical Studies (14)]. Twelve of these subjects (28%) were 1 to 6 years of age; 13 subjects (30%) were 7 to 12 years of age; and 18 subjects (42%) were 13 to 17 years of age. Pharmacokinetic parameters were evaluated for 28 of these subjects who were treated with REBINYN 40 IU/kg [seeClinical Pharmacology (12.3)].
No difference in the safety profile of REBINYN was observed between previously treated pediatric subjects and adult subjects. Body weight-adjusted clearance was higher for pediatric subjects than for adult subjects. Fixed doses were studied in the clinical trials and no dose adjustment was required for pediatric subjects.
Twenty-eight of the forty-three previously treated pediatric subjects (1 to 17 years old) were treated with REBINYN for 137 bleeding episodes. Results are provided in Table 4.
Table 4: Efficacy in treatment of bleeding episodes in pediatric subjects by age
≤ 6 years
7-12 years
13-17 years
New bleeding episodes
n=11
n=31
n=95*
Efficacy assessment**
Excellent or good
10 (91%)
29 (94%)
91 (97%)
Moderate or poor
1 (9%)
2 (6%)
3 (3%)
Number of injections to treat a bleeding episode
1 injection
9 (82%)
27 (87%)
78 (82%)
2 injections
1 (9%)
4 (13%)
12 (13%)
> 2 injections
1 (9%)
-
5 (5%)
*Efficacy assessment was missing for one bleeding episode.
**Efficacy assessment [Response] was assessed according to a four-point scale using:
Excellent: Abrupt pain relief and/or clear improvement in objective signs of bleeding within 8 hours after a single injection; Good: Noticeable pain relief and/or improvement in signs of bleeding within 8 hours after a single injection;
Moderate: Probable or slight beneficial effect within the first 8 hours after the first injection but requiring more than one injection within 8 hours;
Poor: No improvement, or worsening of symptoms within 8 hours after the second of two injections.
Animals administered repeat doses of REBINYN showed accumulation of PEG in the choroid plexus [see Animal Toxicology and/or Pharmacology (13.2)]. The potential clinical implications of these animal findings are unknown. No adverse neurologic effects of PEG have been reported in infants, children, and adolescents exposed to REBINYN during clinical trials. The potential consequences of long term exposure have not been fully evaluated [see Section 6.3].
8.5 Geriatric Use
Clinical studies of REBINYNdid not include sufficient numbers of subjects age 65 and over to determine whether or not they respond differently than younger subjects.
Animals administered repeat doses of REBINYN showed accumulation of PEG in the choroid plexus [see Animal Toxicology and/or Pharmacology (13.2)]. The potential clinical implications of these animal findings are unknown. No adverse neurologic effects of PEG have been reported in adults exposed to REBINYN during clinical trials, however use in older adults with baseline cognitive dysfunction has not been fully evaluated [see Section 6.3].
-
11 DESCRIPTION
REBINYN is a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution with the provided histidine diluent for intravenous infusion. After reconstitution, the solution appears as a clear and colorless liquid, free from visible particles and contains the following excipients per mL: sodium chloride, 2.34 mg; histidine, 3.10 mg; sucrose, 10 mg; mannitol, 25 mg; polysorbate 80, 0.05 mg. REBINYN is available in single-use vials containing the labeled amount of Factor IX activity, expressed in IU. Each vial contains nominally 500 IU, 1000 IU or 2000 IU. REBINYN potency is assigned using an in vitro, activated partial thromboplastin time (aPTT)-based, one-stage clotting assay calibrated against the World Health Organization (WHO) international standard for Factor IX concentrates. REBINYN contains no preservatives.
REBINYN is a purified recombinant human Factor IX (rFIX) with a 40 kilodalton (kDa) polyethylene-glycol (PEG) conjugated to the protein. The 40 kDa PEG group is selectively attached to specific -N-linked glycans in the rFIX activation peptide, with mono-PEGylated rFIX as the predominant form of REBINYN. The rFIX protein in REBINYN consists of a gamma-carboxylated (Gla) domain, two EGF-like (epidermal growth factor) domains, an activation peptide (which is cleaved off upon activation), and a protease domain. Once activated, the resulting rFIX has structural and functional properties similar to those of endogenous activated Factor IX. The primary amino acid sequence in REBINYN is identical to the Thr148 allelic form of human plasma-derived Factor IX and consists of 415 amino acids. The average molecular weight of REBINYN is approximately 98 kDa and the molecular weight of the protein moiety alone is 56 kDa. The nominal specific activity of REBINYN is 152 IU/mg protein.
REBINYN is produced by recombinant DNA technology in Chinese Hamster Ovary (CHO) cells. No additives of human or animal origin are used in the cell culture, purification, conjugation, or formulation of REBINYN. The rFIX protein is purified by a series of chromatographic steps, including an affinity chromatography step using a monoclonal antibody (produced in CHO cells), to selectively isolate rFIX from the cell culture medium. The production process includes two dedicated viral clearance steps, namely a detergent treatment step for inactivation and a 20 nm filtration step for removal of viruses. The conjugation of the PEG-group is done by an enzymatic reaction during the purification process, followed by final purification of REBINYN.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Patients with hemophilia B are deficient in coagulation Factor IX, which is required for effective hemostasis. Treatment with REBINYN temporarily replaces the missing coagulation Factor IX.
The Factor IX in REBINYN is conjugated to a 40-kDa polyethylene glycol molecule, which slows down its removal from the blood circulation.
12.2 Pharmacodynamics
The administration of REBINYN increases plasma levels of Factor IX and can temporarily correct the coagulation defect in hemophilia B patients, as reflected by a decrease in aPTT.
12.3 Pharmacokinetics
Pharmacokinetic (PK) parameters of REBINYN were evaluated in previously treated subjects, including a subset of subjects in the adult/adolescent trial and all subjects in the main phase of the pediatric trial [see Clinical Studies (14)]. PK samples were collected prior to dosing and at multiple time points up to 168 hours after dosing. The analysis of plasma samples was conducted using the one-stage clotting assay.
Steady state pharmacokinetic parameters for adolescents and adults following once-weekly prophylactic treatment of REBINYN 40 IU/kg are shown in Table 5.
Table 5: Steady-state pharmacokinetic parameters of REBINYN (40 IU/kg) in adolescents and adults (geometric mean (CV))
PK Parameter
13-17 years
N=3
≥ 18 years
N=6
Half-life (hours)
103.1 (14.2)
114.9 (9.7)
Incremental Recovery30min (IU/dL per IU/kg)
1.82 (28.2)
1.92 (19.6)
AUC0-168 (IU*hours/dL)
9072 (22)
9280 (15)
Clearance (mL/hour/kg)
0.4 (16.7)
0.4 (11.4)
Mean residence time (hours)
144.4 (15.3)
158.1 (9.6)
Vss (mL/kg)
60.5 (31.1)
65.8 (11.9)
Factor IX activity 168 h after dosing (%)
28.9 (18.6)
32.4 (17.1)
Abbreviations: AUC = area under plasma concentration-time curve; Vss= volume of distribution at steady state; CV=coefficient of variation.
The mean steady state pre-dose trough levels and post-dose peak levels across the clinical trials for all previously treated subjects are shown in Table 6.
Table 6: Factor IX peak and trough levels of REBINYN (40 IU/kg) by age at steady state
≤ 6 years
N=12
7-12 years
N=13
13-17 years
N=9
≥18years N=20
Mean Factor IX peak level (%) (95% CI)
65.5
(60.6; 70.7)71.4
(66.3; 77.0)
82.8
(70.7; 96.9)97.9
(87.7; 109.3)
Mean Factor IX trough level* (%) (95% CI)
Min, Max**
15.4
(13.2; 17.9)
9.2; 24.5
18.7
(16.2; 21.6)
8.3; 28.3
23.7
(19.9; 28.2)
18.6; 34.6
29.3
(26.0; 33.0)
21.3; 42.2
* Factor IX activity from samples collected at clinical site visits just prior to administration of next weekly dose
**Individual geometric mean trough values
Single-dose pharmacokinetic parameters of REBINYN in children, adolescents and adults are listed in Table 7.
Table 7: Single Dose Pharmacokinetic Parameters of REBINYN (40 IU/kg) in children, adolescents and adults (geometric mean (CV))
PK Parameter
≤ 6 years
N=12
7-12 years
N=13
13-17 years
N=3
≥18 years
N=6
Half-life (hours)
69.6 (15.8)
76.3 (25.5)
89.4 (24.1)
83.0 (22.5)
Incremental Recovery30min (IU/dL per IU/kg)
1.51 (7.31)
1.59 (16.2)
1.96 (14.7)
2.34 (11.3)
AUCinf (IU*h/dL)
4617 (14)
5618 (19)
7986 (35)
9063 (16)
Clearance (mL/hour/kg)
0.8 (13.0)
0.6 (21.9)
0.5 (30.4)
0.4 (14.7)
Mean residence time (hours)
95.4 (15.3)
105.1 (24.2)
124.2 (24.4)
115.5 (21.8)
Vss (mL/kg)
72.3 (14.8)
68.3 (21.7)
58.6 (7.8)
47.0 (15.9)
Factor IX activity 168 h after dosing (%)
8.4 (16.3)
10.9 (18.9)
14.6 (59.6)
16.8 (30.6)
Abbreviations: AUC = area under plasma concentration-time curve; Vss = volume of distribution at steady state; CV = coefficient of variation.
Pharmacokinetics were investigated in 9 subjects in the adult/adolescent trial, of which 5 were normal weight (body mass index (BMI) 18.5 to 24.9 kg/m2) and 4 were overweight (BMI 25 to <29.9 kg/m2). The pharmacokinetic parameters were not affected by BMI.
The Factor IX activity following 80 IU/kg infusion in major surgery is shown in Table 8.
Table 8: Factor IX activity following 80 IU/kg bolus for major surgery
30 minutes
8 hours1
24 hours1
48 hours2
N=13
N=12
N=12
N=7
Factor IX activity (%)
Median
(Range)
143
(123-224)
138
(101-175)
112
(62-146)
73
(40-110)
1 Excludes one subject with no Factor IX activity measurement obtained.
2 Excludes two subjects with no Factor IX activity measurement obtained and additionally 4 subjects re-dosed prior to second day after surgery for whom the Factor IX activity at 24 hours were 84%, 112%, 131% and 134%. The 48 hours measurement reflects a measurement on the 2nd day after surgery (range 47-57 hours).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals to evaluate the carcinogenic or genotoxic potential of REBINYN, or studies to determine the effects of REBINYN on fertility, have not been performed.
13.2 Animal Toxicology and/or Pharmacology
REBINYN was intraveneously administered in repeat-dose toxicity studies in immune-deficient rats (40-1200 IU/kg/week for 26 weeks) and immune-competent monkeys (350-3750 IU/kg/week for four weeks). Accumulation of the 40-kDa polyethylene-glycol (PEG) was detected by immunohistochemical staining in epithelial cells of the choroid plexus in the brain of the majority of animals. This finding was not associated with morphological changes or abnormal clinical signs.
-
14 CLINICAL STUDIES
Four multicenter, non-controlled trials were conducted to evaluate the safety and efficacy of REBINYN in routine treatment, on-demand treatment and control of bleeding episodes, and perioperative management in previously treated male patients with hemophilia B (Factor IX activity ≤ 2%). Previously treated patients were defined as patients receiving treatment with other Factor IX products for ≥150 exposure days for adolescents and adults, and ≥50 exposure days for pediatric patients. The key exclusion criteria across trials included known or suspected hypersensitivity to trial or related products, known history of Factor IX inhibitors or current inhibitor ≥0.6 BU, HIV-positive with a viral load ≥400,000 copies/mL or CD4+ lymphocyte count ≤200/μL, additional congenital or acquired coagulation disorders, previous arterial thrombotic events, and recipients of immune modulating or chemotherapeutic medication.
The efficacy evaluation included 105 subjects: 62 adults (18 to 65 years old), 18 adolescents (13 to 17 years old), and 25 children (1 to 12 years old).
- Adult/adolescent trial: The trial included 74 adolescent and adult previously treated patients. There were two routine treatment arms, with single-blind randomization to either 10 IU/kg or 40 IU/kg once-weekly for approximately 52 weeks, and an open-label on-demand treatment arm for approximately 28 weeks.
- Surgery trial: The surgery trial included 13 previously treated adolescent and adult patients who received one infusion of REBINYN 80 IU/kg on the day of surgery, and post-operatively received infusions of 40 IU/kg, at the investigator’s discretion, for up to 3 weeks after surgery.
- Adult/adolescent extension trial: There were 71 subjects from the adult/adolescent trial and surgery trial who continued routine treatment or on-demand treatment with REBINYN in an open-label extension trial, with the possibility to switch regimens during the trial.
- Pediatric trial: The main phase of the pediatric trial included 25 pediatric previously treated patients (1-12 years old) in which subjects received routine treatment with REBINYN 40 IU/kg once-weekly for approximately 52 weeks.
Treatment of Bleeding Episodes
A total of 597 bleeding episodes were reported in 79 out of 105 subjects in the clinical program in previously treated patients. Bleeding episodes were treated with REBINYN at 40 IU/kg for minor or moderate bleeds or 80 IU/kg for major bleeds, with additional doses of 40 IU/kg as needed. The median dose to treat a bleeding episode was 42.3 IU/kg.
An overall assessment of efficacy was performed by the subject (for home treatment) or the study site investigator (for treatment under medical supervision) using a 4-point scale of excellent, good, moderate, or poor. The overall success rate (defined as excellent or good) for treatment of bleeding episodes was 93.2% as shown in Table 9.
The success rate and dose needed for treatment of bleeding episodes were independent of the location of the bleeding. The success rate for treatment of bleeding episodes was also independent of whether the bleed was traumatic or spontaneous.
Table 9: Efficacy in treatment of bleeding episodes in previously treated patients
New Bleeding Episodes
n = 597
Efficacy assessment*
Excellent or Good
551 (93%)
Moderate or Poor
40 (7%)
Number of injections to treat a bleeding episode
1 injection
521 (87%)
2 injections
60 (10%)
>2 injections
16 (3%)
*Efficacy assessment was based on 591 evaluated bleeding episodes (data missing for six bleeding episodes). Efficacy was assessed according to a four-point scale using:
Excellent: Abrupt pain relief and/or clear improvement in objective signs of bleeding within 8 hours after a single injection; Good: Noticeable pain relief and/or improvement in signs of bleeding within 8 hours after a single injection;
Moderate: Probable or slight beneficial effect within the first 8 hours after the first injection but requiring more than one injection within 8 hours;
Poor: No improvement, or worsening of symptoms within 8 hours after the second of two injections.
In the on-demand arm there were 143 bleeding episodes in 14 of 15 subjects. The overall success rate was 95.1% (135 of 142 evaluated bleeds). A total of 120 bleeds (83.9%) of the 143 bleeding episodes were treated with one injection, and 20 (14.0%) were treated with two injections.
Perioperative Management
In the surgery trial, the efficacy analysis of REBINYN in perioperative management included 13 surgical procedures of which 9 were major and performed in 13 previously treated adolescent and adult patients. The procedures included 9 orthopedic, 1 gastrointestinal and 3 in the oral cavity.
The hemostatic effect during surgery was evaluated on a four-point scale of excellent, good, moderate, or poor. The intraoperative hemostatic effect was rated as excellent or good for the 13 surgeries, for a success rate of 100%. A pre-operative dose of 80 IU/kg REBINYN was effective, and no subjects required additional doses on the day of surgery. The median number of additional 40 IU/kg doses in the post-operative period was 2.0 for Days 1 to 6, 1.5 for Days 7-13, and 3.0 for Days 1 to 13. The mean total consumption of REBINYN in the pre- and post-operative period was 241 IU/kg (range: 81 to 460 IU/kg). There was no unexpected postoperative bleeding.
Three additional major surgeries and 18 minor surgery procedures were evaluated in the extension trial for REBINYN in previously treated patients. The hemostatic effect during major and minor surgery was confirmed with a success rate of 100%.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- REBINYN is supplied in packages comprised of a single-use vial containing nominally 500, 1000, or 2000 IU of Factor IX potency; a MixPro® pre-filled diluent syringe containing 10 mM histidine solution (1.6 mg/mL), and a sterile vial adapter with 25 micrometer filter, which serves as a needleless reconstitution device.
- The actual Factor IX potency in IU is stated on each REBINYN carton and vial.
Table 10: REBINYN Presentations
Presentation (Nominal Product Strength; IU)
Cap Color Indicator
Carton NDC Number
Components
500
Red
NDC 0169 7905 01
- REBINYN in single-use vial [NDC 0169 7955 11]
- Pre-filled histidine diluent in syringe, 4 mL [NDC 0169 7009 98]
- Vial adapter
1000
Green
NDC 0169 7901 01
- REBINYN in single-use vial [NDC 0169 7911 11]
- Pre-filled histidine diluent in syringe, 4 mL [NDC 0169 7009 98]
- Vial adapter
2000
Yellow
NDC 0169 7902 01
- REBINYN in single-use vial [NDC 0169 7922 11]
- Pre-filled histidine diluent in syringe, 4 mL [NDC 0169 7009 98]
- Vial adapter
- The REBINYN vials are made of glass, closed with a chlorobutyl rubber stopper (not made with natural rubber latex), and sealed with an aluminum cap.
- The pre-filled diluent syringes are made of glass, with a siliconised bromobutyl rubber plunger (not made with rubber latex).
- The closed vials and pre-filled diluent syringes are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Storage and Handling
- Store REBINYN in the original package in order to protect from light.
- Store REBINYN under refrigeration at a temperature of 36°F-46°F (2°C – 8°C) for up to 24 months from the date of manufacture until the expiration date stated on the label.
- REBINYN may be stored at room temperature not to exceed 86°F (30°C) for up to 6 months within the 24-month time period. Record the date when the product was removed from the refrigerator in the space provided on the outer carton. The total time of storage at room temperature should not exceed 6 months. Do not return the product to the refrigerator.
- Do not use REBINYN after the end of the 6-month period at room temperature storage, or after the expiration date stated on the vial, whichever occurs earlier.
- Do not freeze REBINYN.
- Use REBINYN within 4 hours after reconstitution when stored at room temperature. Store the reconstituted product in the vial.
- Discard any unused reconstituted product stored at room temperature for more than 4 hours.
-
17 PATIENT COUNSELING INFORMATION
- Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Inform patients of the early signs of hypersensitivity reactions including rash, hives, itching, facial swelling, tightness of the chest and wheezing. Advise patients to discontinue use of the product and contact their healthcare provider if these symptoms occur.
- Advise patients to contact their healthcare provider for further treatment and/or assessment if they experience a lack of a clinical response to Factor IX therapy, as in some cases this may be a manifestation of an inhibitor.
- Advise patients to contact their healthcare provider if they experience any thrombotic complications.
- Advise patients to follow the recommendations regarding proper sharps disposal provided in the FDA-approved Instructions for Use.
Version: 1
License Number: 1261
REBINYN® and MixPro® are trademarks of Novo Nordisk A/S.
For Patent Information, refer to: http://novonordisk-us.com/patients/products/product-patents.html
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, Invision-Plus® Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector® is a registered trademark of Vygon.
© 2017 Novo Nordisk
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-844-REB-INYN
Manufactured by:
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsvaerd
-
Patient Package Insert
Patient Product Information
REBINYN (reh-bē-NINE)
Coagulation Factor IX (Recombinant), GlycoPEGylated
Read the Patient Product Information and the Instructions For Use that come with REBINYN before you start taking this medicine and each time you get a refill. There may be new information.
This Patient Product Information does not take the place of talking with your healthcare provider about your medical condition or treatment. If you have questions about REBINYN after reading this information, ask your healthcare provider.
What is the most important information I need to know about REBINYN?
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia treatment center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing REBINYN so that your treatment will work best for you.
What is REBINYN?
REBINYN is an injectable medicine used to replace clotting Factor IX that is missing in patients with hemophilia B. Hemophilia B is an inherited bleeding disorder in all age groups that prevents blood from clotting normally.
REBINYN is used to treat and control bleeding in people with hemophilia B.
Your healthcare provider may give you REBINYN when you have surgery.
Who should not use REBINYN?
You should not use REBINYN if you
- are allergic to Factor IX or any of the other ingredients of REBINYN
- if you are allergic to hamster proteins
If you are not sure, talk to your healthcare provider before using this medicine.
Tell your healthcare provider if you are pregnant or nursing because REBINYN might not be right for you.
What should I tell my healthcare provider before I use REBINYN?
You should tell your healthcare provider if you
- Have or have had any medical conditions.
- Take any medicines, including non-prescription medicines and dietary supplements.
- Are nursing.
- Are pregnant or planning to become pregnant.
- Have been told that you have inhibitors to Factor IX.
How should I use REBINYN?
Treatment with REBINYN should be started by a healthcare provider who is experienced in the care of patients with hemophilia B.
REBINYN is given as an infusion into the vein.
You may infuse REBINYN at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your hemophilia treatment center or healthcare provider. Many people with hemophilia B learn to infuse the medicine by themselves or with the help of a family member.
Your healthcare provider will tell you how much REBINYN to use based on your weight, the severity of your hemophilia B, and where you are bleeding. Your dose will be calculated in international units, IU.
Call your healthcare provider right away if your bleeding does not stop after taking REBINYN.
If your bleeding is not adequately controlled, it could be due to the development of Factor IX inhibitors. This should be checked by your healthcare provider. You might need a higher dose of REBINYN or even a different product to control bleeding. Do not increase the total dose of REBINYN to control your bleeding without consulting your healthcare provider.
Use in children
REBINYN can be used in children. Your healthcare provider will decide the dose of REBINYN you will receive.
If you forget to use REBINYN
If you forget a dose, infuse the missed dose when you discover the mistake. Do not infuse a double dose to make up for a forgotten dose. Proceed with the next infusions as scheduled and continue as advised by your healthcare provider.
If you stop using REBINYN
Do not stop using REBINYN without consulting your healthcare provider.
If you have any further questions on the use of this product, ask your healthcare provider.
What if I take too much REBINYN?
Always take REBINYN exactly as your healthcare provider has told you. You should check with your healthcare provider if you are not sure. If you infuse more REBINYN than recommended, tell your healthcare provider as soon as possible.
What are the possible side effects of REBINYN?
Common Side Effects Include:
- swelling, pain, rash or redness at the location of infusion
- itching
Other Possible Side Effects:
You could have an allergic reaction to coagulation Factor IX products. Call your healthcare provider right away or get emergency treatment right away if you get any of the following signs of an allergic reaction: hives, chest tightness, wheezing, difficulty breathing, and/or swelling of the face.
Your body can also make antibodies called “inhibitors” against REBINYN, which may stop REBINYN from working properly. Your healthcare provider may need to test your blood for inhibitors from time to time.
You may be at an increased risk of forming blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider if you have chest pain, difficulty breathing, leg tenderness or swelling.
These are not all of the possible side effects from REBINYN. Ask your healthcare provider for more information. You are encouraged to report side effects to FDA at 1-800-FDA-1088.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
What are the REBINYN dosage strengths?
REBINYN comes in three different dosage strengths. The actual number of international units (IU) of Factor IX in the vial will be imprinted on the label and on the box. The three different strengths are as follows:
Cap Color Indicator
Nominal Strength
Red
500 IU per vial
Green
1000 IU per vial
Yellow
2000 IU per vial
Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
How should I store REBINYN?
Prior to Reconstitution (mixing the dry powder in the vial with the diluent):
Store in original package in order to protect from light. Do not freeze REBINYN.
REBINYN vials can be stored in the refrigerator (36-46°F [2°C – 8°C]) for up to 24 months until the expiration date, or at room temperature (up to 86°F [30°C]) for a single period not more than 6 months.
If you choose to store REBINYN at room temperature:
- Note the date that the product is removed from refrigeration on the box.
- The total time of storage at room temperature should not be more than 6 months. Do not return the product to the refrigerator.
- Do not use after 6 months from this date or the expiration date listed on the vial, whichever is earlier.
Do not use this medicine after the expiration date which is on the outer carton and the vial. The expiration date refers to the last day of that month.
After Reconstitution:
The reconstituted (the final product once the powder is mixed with the diluent) REBINYN should appear clear without visible particles.
The reconstituted REBINYN should be used immediately.
If you cannot use the reconstituted REBINYN immediately, it should be used within 4 hours when stored at or below 86ºF (30°C). Store the reconstituted product in the vial.
Keep this medicine out of the sight and out of reach of children.
What else should I know about REBINYN and hemophilia B?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use REBINYN for a condition for which it is not prescribed. Do not share REBINYN with other people, even if they have the same symptoms that you have.
For more information about REBINYN, please call Novo Nordisk at 1-844-REB-INYN.
Revised: 05/2017
REBINYN® is a trademark of Novo Nordisk A/S.
For Patent Information, refer to:
http://novonordisk-us.com/patients/products/product-patents.html
© 2017 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd, Denmark
For information about REBINYN contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
Instructions on how to use REBINYN® MixPro®
READ THESE INSTRUCTIONS CAREFULLY BEFORE USING REBINYN.
REBINYN is supplied as a powder. Before infusion (administration) it must be mixed (reconstituted) with the liquid diluent supplied in the syringe. The liquid diluent is a histidine solution. The mixed REBINYN must be infused into your vein (intravenous infusion). The equipment in this package is designed to mix and infuse REBINYN.
You will also need an infusion set (tubing and butterfly needle), sterile alcohol swabs, gauze pads, and bandages.
Don’t use the equipment without proper training from your doctor or nurse.
Always wash your hands and ensure that the area around you is clean.
When you prepare and infuse medication directly into the veins, it is important to use a clean and germ free (aseptic) technique. Improper technique can introduce germs that can infect the blood.
Don’t open the equipment until you are ready to use it.
Don’t use the equipment if it has been dropped, or if it is damaged. Use a new package instead.
Don’t use the equipment if it is expired. Use a new package instead.The expiration date is printed on the outer carton and on the vial, the vial adapter and the pre-filled syringe.
Don’t use the equipment if you suspect it is contaminated. Use a new package instead.
Don’t dispose of any of the items until after you have infused the mixed solution.
The equipment is for single use only.
Content
The package contains:
Vial with REBINYN powder
Vial adapter
Pre-filled syringe with diluent
Plunger rod (placed under the syringe)
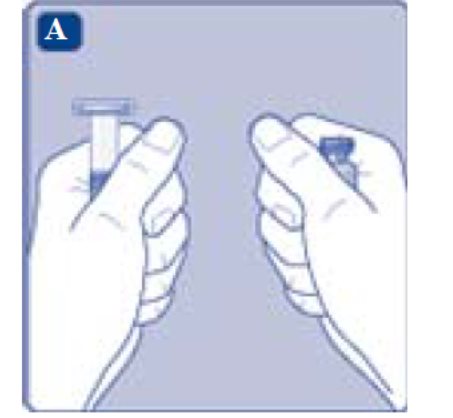
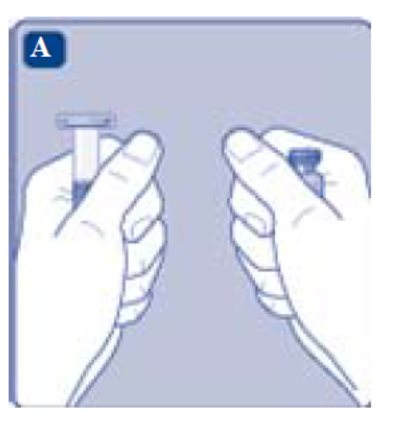
1. Prepare the vial and the syringe
- Take out the number of REBINYN® packages you need.
- Check the expiry date.
- Check the name, strength and color of the package, to make sure it contains the correct product.
- Wash your hands and dry them properly using a clean towel or air dry.
- Take the vial, the vial adapter and the pre-filled syringe out of the carton. Leave the plunger rod untouched in the carton.
- Bring the vial and the pre-filled syringe to room temperature. You can do this by holding them in your hands until they feel as warm as your hands.
- Remove the plastic cap from the vial. If the plastic cap is loose or missing, don’t use the vial.
- Wipe the rubber stopper with a sterile alcohol swab and allow it to air dry for a few seconds before use to ensure that it is as germ free as possible.
- Don’t touch the rubber stopper with your fingers as this can transfergerms.
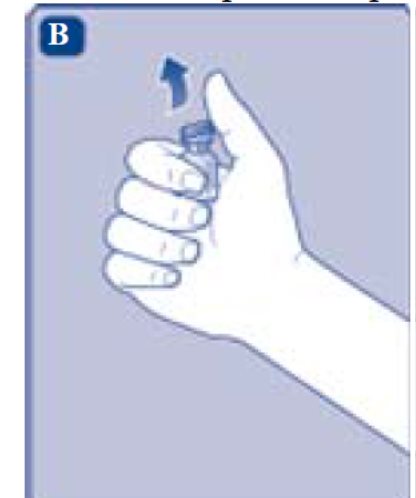
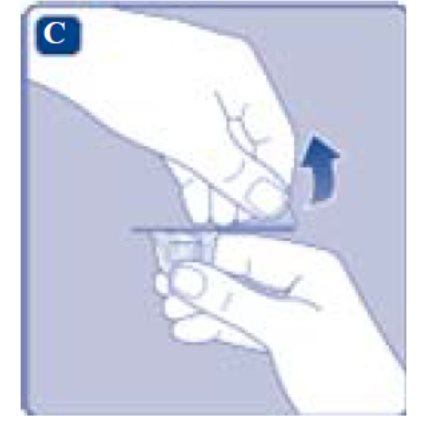
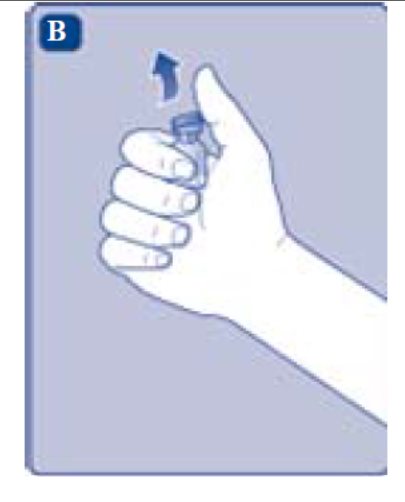
2. Attach the vial adapter
- Remove the protective paper from the vial adapter.
Don’t take the vial adapter out of the protective cap with your fingers. If you touch the spike on the vial adapter germs from your fingers can be transferred.
If the protective paper is not fully sealed or if it is broken, don’t use the vial adapter.
- Place the vial on a flat and solid surface.
- Turn over the protective cap, and snap the vial adapter onto the vial.
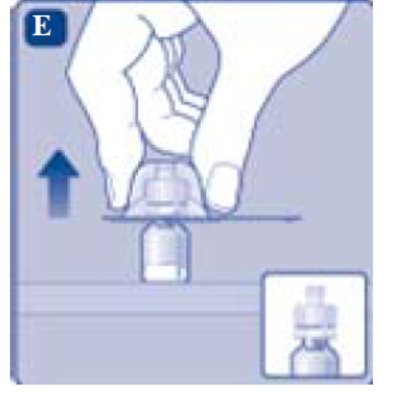
- Once attached, don’t remove the vial adapter from the vial.
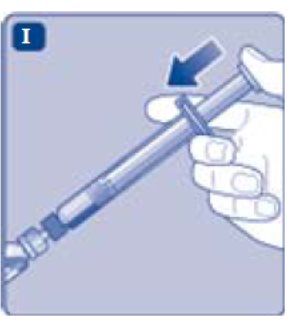
- Lightly squeeze the protective cap with your thumb and index finger as shown. Remove the protective cap from the vial adapter.
- Don’t lift the vial adapter from the vial when removing the protective cap.
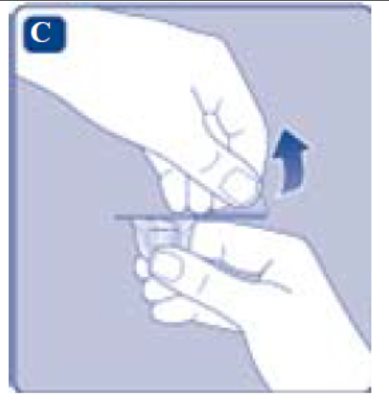
3. Attach the plunger rod and the syringe
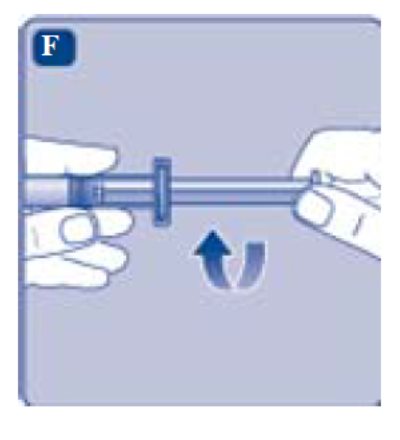
- Grasp the plunger rod by the wide top end and take it out of the carton. Don’t touch the sides or the thread of the plunger rod. If you touch the sides or the thread germs from your fingers can be transferred.
- Immediately connect the plunger rod to the syringe by turning it clockwise into the rubber plunger inside the pre-filled syringe until resistance is felt.
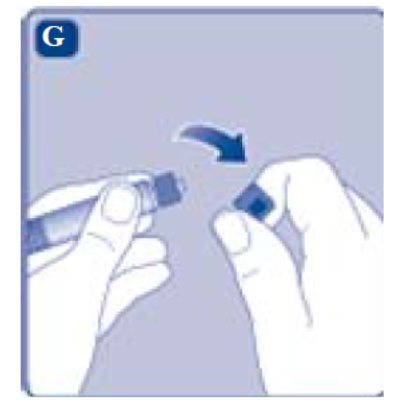
- Remove the syringe cap from the pre-filled syringe by bending it down until the perforation breaks.
- Don’t touch the syringe tip under the syringe cap. If you touch the syringe tip germs from your fingers can be transferred.
- If the syringe cap is loose or missing, don’t use the pre-filled syringe.
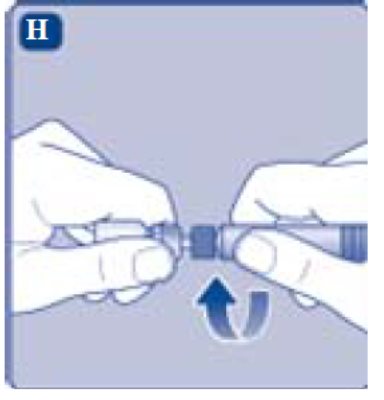
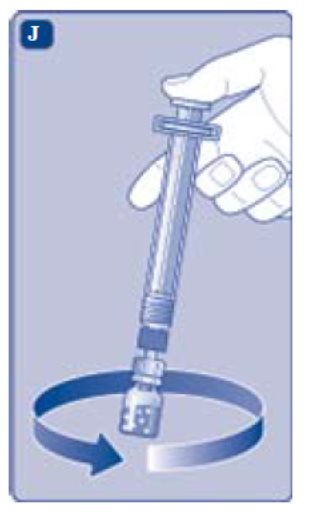
- Screw the pre-filled syringe securely onto the vial adapter until resistance is felt.
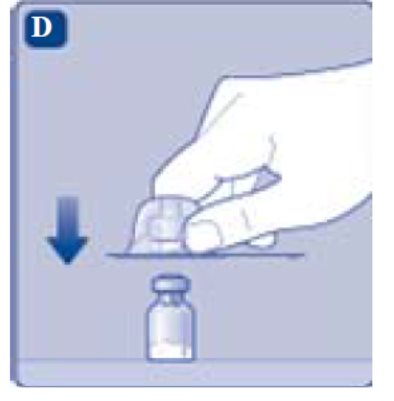
4. Mix the powder with the diluent
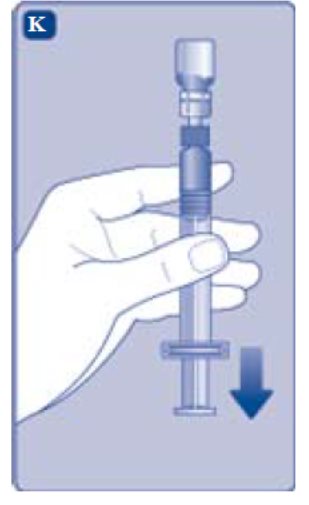
- Hold the pre-filled syringe slightly tilted with the vial pointing downwards.
- Push the plunger rod to inject all the diluent into the vial.
- Keep the plunger rod pressed down and swirl the vial gently until all the powder is dissolved.
- Don’t shake the vial as this will cause foaming.
- Check the mixed solution. It must be clear and colorless. If you notice visible particles or discoloration, don’t use it. Use a new package instead.
REBINYN is recommended to be used immediately after it is mixed.
If you cannot use the mixed REBINYN solution immediately, it should be used within 4 hours when stored at room temperature at or below 86°F (30°C). Store the reconstituted product in the vial.
Do not freeze mixed REBINYN solution or store it in syringes.
- Keep mixed REBINYN solution out of direct light.
- If your dose requires more than one vial, repeat step A to J with additional vials, vial adapters and pre-filled syringes until you have reached your required dose.
- Keep the plunger rod pushed completely in.
- Turn the syringe with the vial upside down.
- Stop pushing the plunger rod and let it move back on its own while the mixed solution fills the syringe.
- Pull the plunger rod slightly downwards to draw the mixed solution into the syringe.
- In case you only need part of the entire vial, use the scale on the syringe to see how much mixed solution you withdraw, as instructed by your doctor or nurse.
- While holding the vial upside down, tap the syringe gently to let any air bubbles rise to the top.
- Push the plunger rod slowly until all air bubbles are gone.
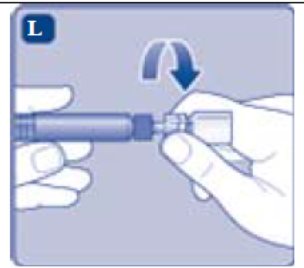
- Unscrew the vial adapter with the vial.
Don’t touch the syringe tip. If you touch the syringe tip germs from your fingers can be transferred.
Caution: The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus® Junior®, Bionector®).
The use of these needleless connectors can damage the connector and affect administration.
To administer REBINYN through incompatible needleless connectors, withdraw reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
If you have encountered any problems with attaching the pre-filled histidine diluent syringe to any Luer‐lock compatible device, please contact Novo Nordisk at (844) 303-4448.
- 5. Infuse the mixed solution
REBINYN is now ready to infuse into your vein.
- Do not mix REBINYN with any other intravenous infusions or medications.
- Infuse the mixed solution slowly over 1 to 4 minutes as instructed by your doctor or nurse.
Infusing the solution via a central venous access device (CVAD) such as a central venous catheter or subcutaneous port:
- Use a clean and germ free (aseptic) technique. Follow the instructions for proper use for your connector and central venous access device in consultation with your doctor or nurse.
- Infusing into a CVAD may require using a sterile 10 mL plastic syringe for withdrawal of the mixed solution and infusion.
-
If necessary, use 0.9% Sodium Chloride Injection, USP to flush the CVAD line before or after REBINYN infusion.
The peel-off label found on the REBINYN vial can be used to record the lot number.
Disposal
- After infusion, safely dispose of all unused REBINYN solution, the syringe with the infusion set, the vial with the vial adapter, and other waste materials in an appropriate container for throwing away medical waste.
- Don’t throw it out with the ordinary household trash.
Don’t disassemble the vial and vial adapter before disposal.
Don’t reuse the equipment.
Important information
Contact your healthcare provider or local hemophilia treatment center if you experience any problems.
For full Prescribing Information please read the other insert included in this package.
REBINYN® and MixPro® are trademarks of Novo Nordisk A/S.
For Patent Information, refer to: http://novonordisk-us.com/patients/products/product-patents.html
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, Invision-Plus®Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector is a registered trademark of Vygon
© 2017 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about REBINYN contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
License No: 1261
- Revised: 05/2017
- PRINCIPAL DISPLAY PANEL
- Principal Display Panel
-
Principal Display Panel
NDC 0169 7902 01 List: 790201 2000 IU
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
2000 IU
Intravenous use, after reconstitution.
Single use only.
Contains no preservatives.
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
NDC 0169 7955 11
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
500 IU
Store in a refrigerator 36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
NDC 0169 7911 11
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
1000 IU
Store in a refrigerator 36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
NDC 0169 7922 11
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
2000 IU
Store in a refrigerator 36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
NDC 0169 7009 98
Histidine (10 mM Solution) 4 mL
For reconstitution of Tradename
Store in a refrigerator 36°F to 46°F (2°C to 8°C)
Do not freeze
Rx Only
Single Use Only
-
INGREDIENTS AND APPEARANCE
REBINYN
(coagulation factor ix (recombinant), glycopegylated) kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0169-7905 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7905-01 1 in 1 KIT; Type 0: Not a Combination Product 12/01/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 REBINYN
(coagulation factor ix (recombinant), glycopegylated) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0169-7955 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 2.34 mg in 1 mL SUCROSE (UNII: C151H8M554) 10 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 25 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.05 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7955-11 4 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Part 2 of 2 REBINYN
(coagulation factor ix (recombinant), glycopegylated) solutionProduct Information Item Code (Source) NDC: 0169-7009 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7009-98 4 mL in 1 SYRINGE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 REBINYN
(coagulation factor ix (recombinant), glycopegylated) kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0169-7901 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7901-01 1 in 1 KIT; Type 0: Not a Combination Product 12/01/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 REBINYN
(coagulation factor ix (recombinant), glycopegylated) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0169-7911 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 1000 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 2.34 mg in 1 mL SUCROSE (UNII: C151H8M554) 10 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 25 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.05 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7911-11 4 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Part 2 of 2 REBINYN
(coagulation factor ix (recombinant), glycopegylated) solutionProduct Information Item Code (Source) NDC: 0169-7009 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7009-98 4 mL in 1 SYRINGE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 REBINYN
(coagulation factor ix (recombinant), glycopegylated) kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0169-7902 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7902-01 1 in 1 KIT; Type 0: Not a Combination Product 12/01/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 REBINYN
(coagulation factor ix (recombinant), glycopegylated) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 0169-7922 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 2000 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 2.34 mg in 1 mL SUCROSE (UNII: C151H8M554) 10 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 25 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.05 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7922-11 4 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Part 2 of 2 REBINYN
(coagulation factor ix (recombinant), glycopegylated) solutionProduct Information Item Code (Source) NDC: 0169-7009 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-7009-98 4 mL in 1 SYRINGE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125611 12/01/2017 Labeler - Novo Nordisk (622920320) Establishment Name Address ID/FEI Business Operations Novo Nordisk A/S 306711800 MANUFACTURE(0169-7905, 0169-7901, 0169-7902, 0169-7955, 0169-7911, 0169-7922, 0169-7009) Establishment Name Address ID/FEI Business Operations Novo Nordisk A/S 305914798 API MANUFACTURE(0169-7905, 0169-7901, 0169-7902, 0169-7955, 0169-7911, 0169-7922, 0169-7009)
Trademark Results [REBINYN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REBINYN 79156280 4714544 Live/Registered |
Novo Nordisk Health Care AG 2014-07-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.