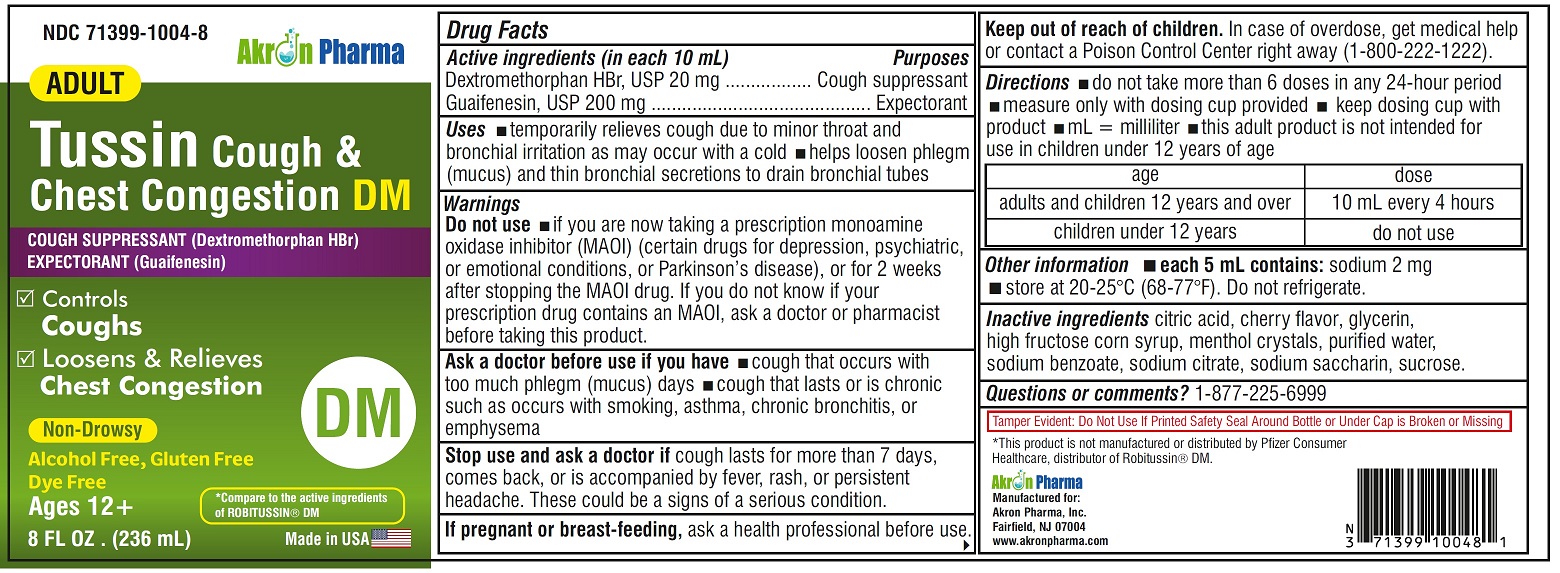
Adult Tussin Cough and Chest Congestion DM by Akron Pharma Inc. / SLV PHARMACEUTICALS LLC Drug Facts
Adult Tussin Cough and Chest Congestion DM by
Drug Labeling and Warnings
Adult Tussin Cough and Chest Congestion DM by is a Otc medication manufactured, distributed, or labeled by Akron Pharma Inc., SLV PHARMACEUTICALS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ADULT TUSSIN COUGH AND CHEST CONGESTION DM- dextromethorphan hbr, guaifenesin liquid
Akron Pharma Inc.
----------
Drug Facts
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- mL= milliliter
- this adult product is not intended for use in children under 12 years of age
- adults and children 12 years and over: 10 mL every 4 hours
- children under 12 years: do not use
Other information
- each 5 mL contains: sodium 2 mg
- store between 20-25ºC(68-77ºF). Do not refrigerate
| ADULT TUSSIN COUGH AND CHEST CONGESTION DM
dextromethorphan hbr, guaifenesin liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Akron Pharma Inc. (067878881) |
| Registrant - SLV PHARMACEUTICALS LLC (081225162) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SLV PHARMACEUTICALS LLC | 081225162 | manufacture(71399-1004) | |
Revised: 1/2024
Document Id: 8b5c5f69-3ad2-44d0-ae85-599b22f4b900
Set id: 0ea4968d-34d6-487a-af0d-ebdc677c22d9
Version: 2
Effective Time: 20240118
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.