PARASODE- artemisia vulgaris root, chelidonium majus whole, punica granatum root bark, spigelia anthelmia whole, krameria lappacea root, arsenic trioxide, iron, oyster shell calcium carbonate, crude, artemisia cina pre-flowering top, salmonella enterica enterica serovar enteritidis, graphite, calomel, sodium chloride, and sodium phosphate, dibasic, heptahydrate liquid
Parasode by
Drug Labeling and Warnings
Parasode by is a Homeopathic medication manufactured, distributed, or labeled by Zorex, OHM Pharma, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
ACTIVE INGREDIENTS
equal amounts of: Artemisia Vulgaris 4X, Chelidonium Majus 4X, Granatum 4X, Spigelia Anthelmia 4X, Ratanhia 4X, Arsenicum Album 6X, Ferrum Metallicum 9X, Calcarea Carbonica 12X, Cina 12X, Gaertner Bacillus 12X, Graphites 12X, Mercurius Dulcis 12X, Natrum Muriaticum 12X, Natrum Phosphoricum 12X, Vermin Nosode 30X, 60X, 200X.
- USES
- DIRECTIONS
- WARNINGS
- INACTIVE INGREDIENTS
- Questions & Comments?
-
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label
Zorex
International™NDC: 81825-0012-1
PARASODE
HOMEOPATHIC
1 fl oz (30 mL) / 20% Alcohol
Rev. 01
"Our Mission
is Excellence
in Nutrition"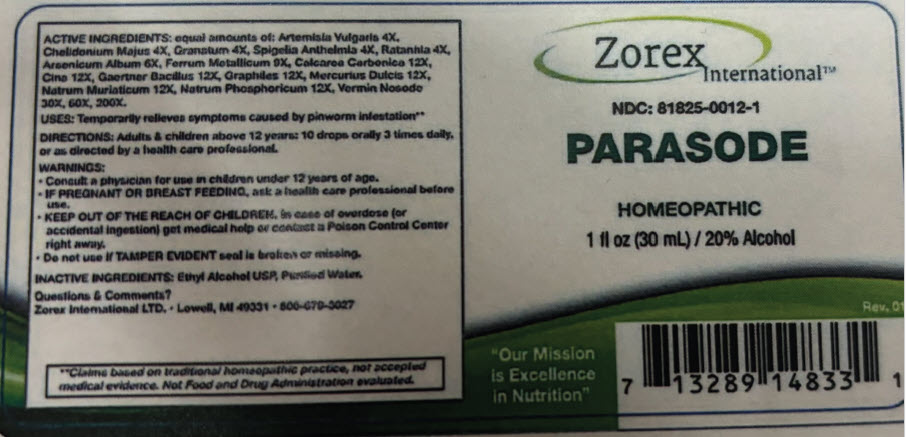
-
INGREDIENTS AND APPEARANCE
PARASODE
artemisia vulgaris root, chelidonium majus whole, punica granatum root bark, spigelia anthelmia whole, krameria lappacea root, arsenic trioxide, iron, oyster shell calcium carbonate, crude, artemisia cina pre-flowering top, salmonella enterica enterica serovar enteritidis, graphite, calomel, sodium chloride, and sodium phosphate, dibasic, heptahydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 81825-0012 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength artemisia vulgaris root (UNII: 32MP823R8S) (artemisia vulgaris root - UNII:32MP823R8S) artemisia vulgaris root 4 [hp_X] in 1 mL chelidonium majus whole (UNII: 7E889U5RNN) (chelidonium majus whole - UNII:7E889U5RNN) chelidonium majus whole 4 [hp_X] in 1 mL punica granatum root bark (UNII: CLV24I3T1D) (punica granatum root bark - UNII:CLV24I3T1D) punica granatum root bark 4 [hp_X] in 1 mL spigelia anthelmia whole (UNII: WYT05213GE) (spigelia anthelmia whole - UNII:WYT05213GE) spigelia anthelmia whole 4 [hp_X] in 1 mL krameria lappacea root (UNII: P29ZH1A35Z) (krameria lappacea root - UNII:P29ZH1A35Z) krameria lappacea root 4 [hp_X] in 1 mL arsenic trioxide (UNII: S7V92P67HO) (arsenic cation (3+) - UNII:C96613F5AV) arsenic trioxide 6 [hp_X] in 1 mL iron (UNII: E1UOL152H7) (iron - UNII:E1UOL152H7) iron 9 [hp_X] in 1 mL oyster shell calcium carbonate, crude (UNII: 2E32821G6I) (oyster shell calcium carbonate, crude - UNII:2E32821G6I) oyster shell calcium carbonate, crude 12 [hp_X] in 1 mL artemisia cina pre-flowering top (UNII: 28M1820ACT) (artemisia cina pre-flowering top - UNII:28M1820ACT) artemisia cina pre-flowering top 12 [hp_X] in 1 mL salmonella enterica enterica serovar enteritidis (UNII: Y3V16D4PV4) (salmonella enterica enterica serovar enteritidis - UNII:Y3V16D4PV4) salmonella enterica enterica serovar enteritidis 12 [hp_X] in 1 mL graphite (UNII: 4QQN74LH4O) (graphite - UNII:4QQN74LH4O) graphite 12 [hp_X] in 1 mL calomel (UNII: J2D46N657D) (calomel - UNII:J2D46N657D) calomel 12 [hp_X] in 1 mL sodium chloride (UNII: 451W47IQ8X) (chloride ion - UNII:Q32ZN48698) sodium chloride 12 [hp_X] in 1 mL sodium phosphate, dibasic, heptahydrate (UNII: 70WT22SF4B) (phosphate ion - UNII:NK08V8K8HR) sodium phosphate, dibasic, heptahydrate 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81825-0012-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 06/27/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/27/2025 Labeler - Zorex (122070786) Establishment Name Address ID/FEI Business Operations OHM Pharma, Inc 030572478 MANUFACTURE(81825-0012)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.