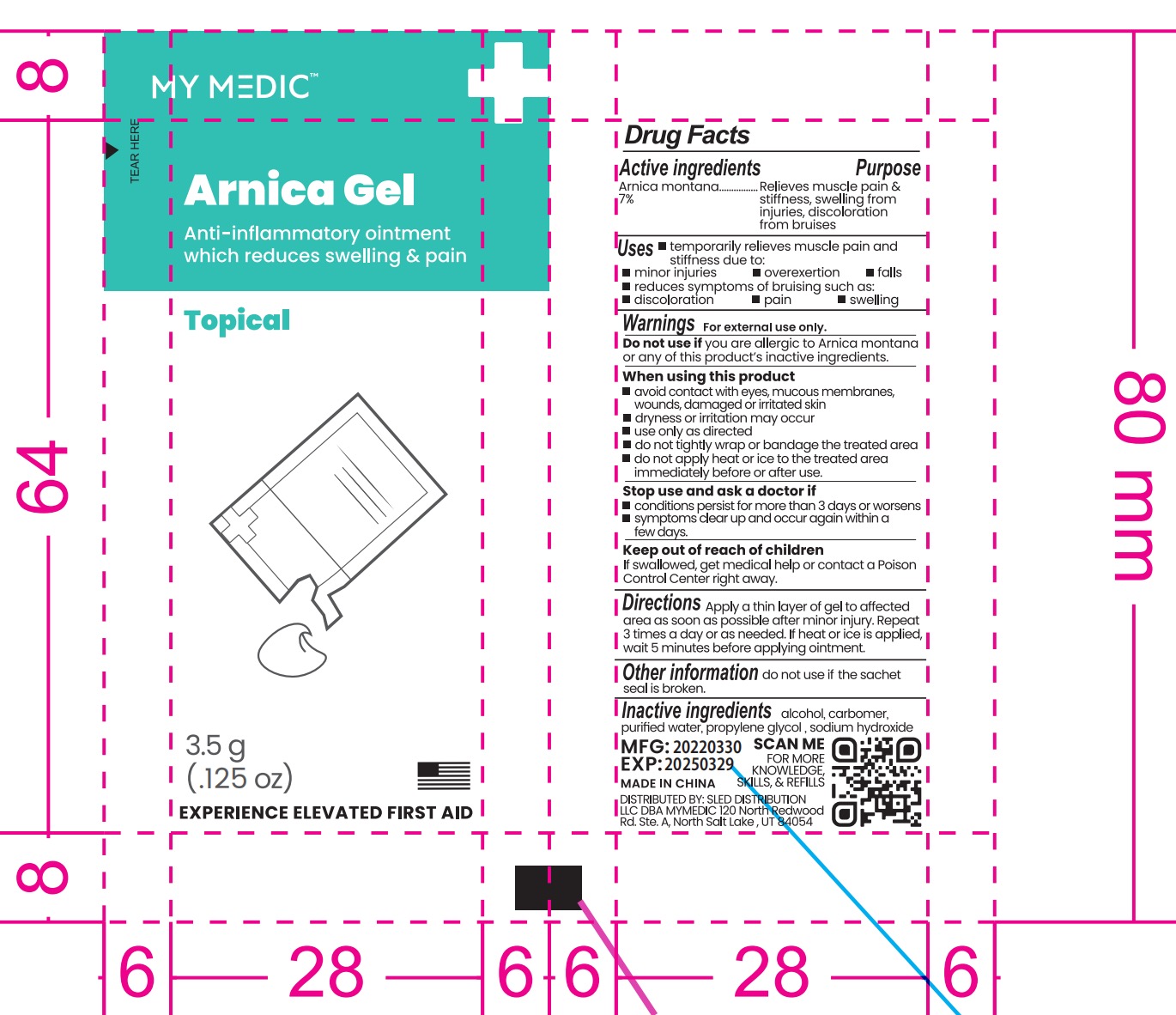
MYMEDIC ARNICA GEL- arnica montana gel
MyMedic Arnica Gel by
Drug Labeling and Warnings
MyMedic Arnica Gel by is a Otc medication manufactured, distributed, or labeled by Nantong Health & Beyond Hygienic Products Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MYMEDIC ARNICA GEL
arnica montana gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43473-304 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER 940 (UNII: 4Q93RCW27E) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size 80mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43473-304-01 3.5 g in 1 POUCH; Type 0: Not a Combination Product 01/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/15/2024 Labeler - Nantong Health & Beyond Hygienic Products Inc. (421280161) Registrant - Nantong Health & Beyond Hygienic Products Inc. (421280161) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 manufacture(43473-304)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.