EXTRALASTING CREAM-TO-POWDER FOUNDATION- octinoxate, titanium dioxide cream
Extralasting by
Drug Labeling and Warnings
Extralasting by is a Otc medication manufactured, distributed, or labeled by New Avon LLC, Avon Products, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
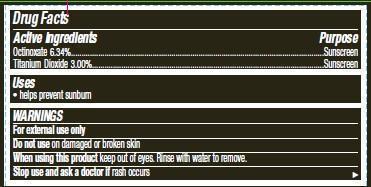
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
apply liberally 15 minutes before sun exposure
children under 6 months of age: ask a doctor
reapply at least every 2 hours
use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients: Propylene Glycol Dicaprylate/Dicaprate, Diisopropyl Dimer Dilinoleate, Microcrystalline Wax/Cire Microcristalline, Nylon-12, Cetyl Ricinoleate, Hydrogenated Coco-Glycerides, Silica, Tribehenin, Punica Granatum Sterols, Polymethyl Methacrylate, Ethylene/Methacrylate Copolymer, Propylparaben, VP/Hexadecene Copolymer, Dimethicone, Ethylparaben, Triethoxycaprylylsilane, Glyceryl Rosinate, Octyldodecyl Myristate, Magnesium Myristate, Butylparaben, Tocopheryl Acetate, Isopropyl Titanium Triisostearate. May Contain: Titanium Dioxide/CI 77891, Iron Oxides, Mica/CI 77019, Bismuth Oxychloride/CI 77163.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXTRALASTING CREAM-TO-POWDER FOUNDATION
octinoxate, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10096-0231 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 63.4 mg in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 30 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10096-0231-2 1 in 1 CARTON 1 NDC: 10096-0231-1 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/15/2010 Labeler - New Avon LLC (080143520) Establishment Name Address ID/FEI Business Operations Avon Products, Inc. 544863277 manufacture(10096-0231)
Trademark Results [Extralasting]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXTRALASTING 85054709 3907636 Live/Registered |
AVON NA IP LLC 2010-06-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.