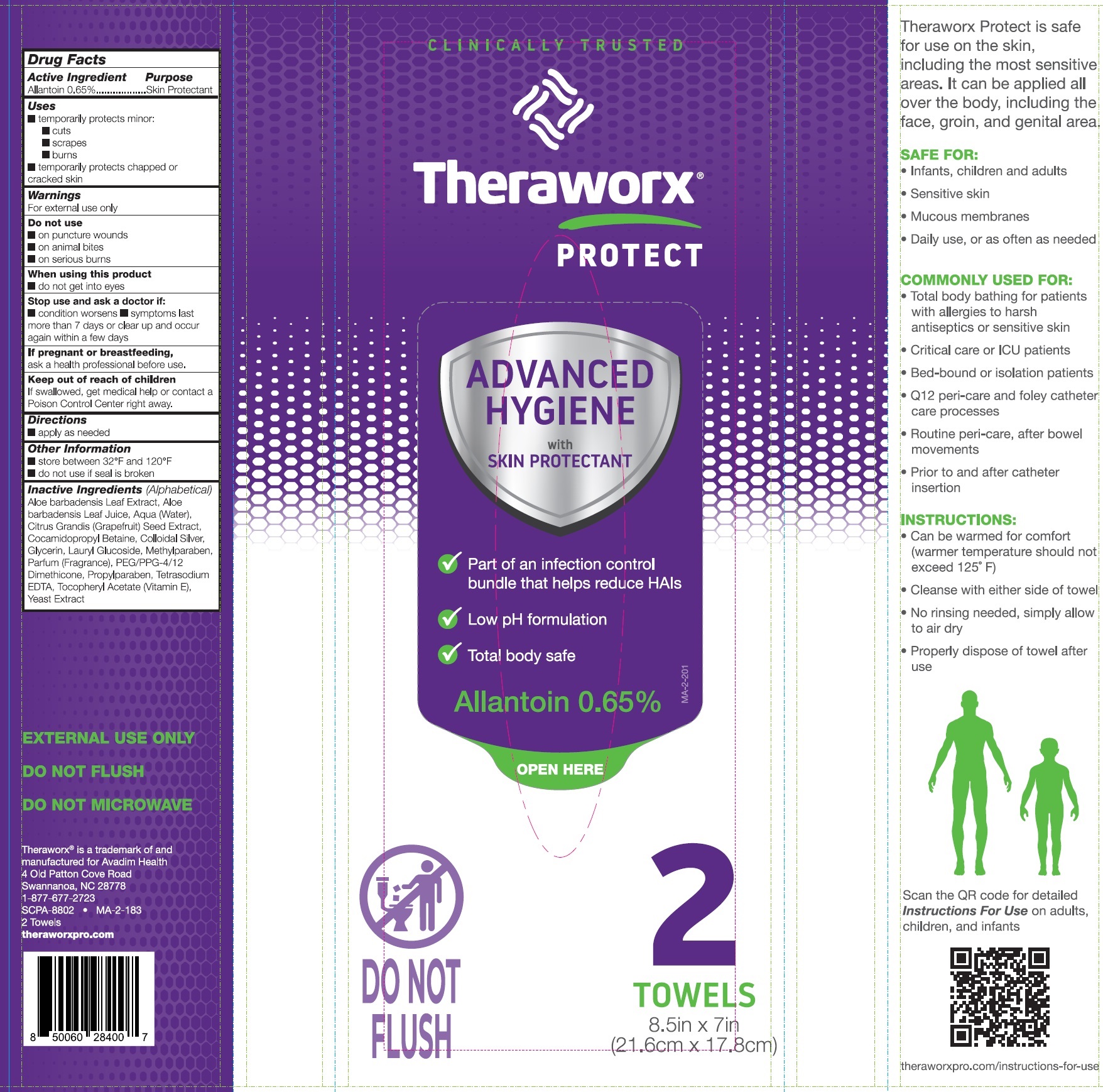
Theraworx Protect Advanced Hygiene
Theraworx Protect Advanced Hygiene by
Drug Labeling and Warnings
Theraworx Protect Advanced Hygiene by is a Otc medication manufactured, distributed, or labeled by AVADIM HOLDINGS, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
THERAWORX PROTECT ADVANCED HYGIENE- allantoin cloth
AVADIM HOLDINGS, INC.
----------
Theraworx Protect Advanced Hygiene
Warnings
For external use only
Inactive Ingredients (Alphabetical)
Aloe barbadensis Leaf Extract, Aloe barbadensis Leaf Juice, Aqua (Water), Citrus Grandis (Grapefruit) Seed Extract, Cocamidopropyl Betaine, Colloidal Silver, Glycerin, Lauryl Glucoside, Methylparaben, Parfum (Fragrance), PEG/PPG-4/12 Dimethicone, Propylparaben, Tetrasodium EDTA, Tocopheryl Acetate (Vitamin E), Yeast Extract
| THERAWORX PROTECT ADVANCED HYGIENE
allantoin cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - AVADIM HOLDINGS, INC. (118512488) |
Revised: 10/2024
Document Id: 254f25ac-0267-c2e8-e063-6394a90a11d5
Set id: 0f280b86-aa2f-6511-e063-6394a90a283f
Version: 2
Effective Time: 20241025
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.