REFRESH PLUS- carboxymethylcellulose sodium solution/ drops
REFRESH PLUS by
Drug Labeling and Warnings
REFRESH PLUS by is a Otc medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
-
Warnings
-
For external use only.
-
To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
-
Do not touch unit-dose tip to eye.
- If solution changes color or becomes cloudy, do not use.
-
For external use only.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NDC: 0023-0403-30
Preservative-free
Refresh
Plus
Lubricant Eye Drops
MOISTURIZING RELIEF
Immediate, soothing relief
for dry eyes. Also recommended
for LASIK dryness*
30 Single-Use Containers
0.01 fl oz (0.4 mL) each Sterile
-
PRINCIPAL DISPLAY PANEL
NDC: 0023-0403-30
Preservative-free
Refresh
Plus
Lubricant Eye Drops
MOISTURIZING RELIEF
Immediate, soothing relief
for dry eyes. Also recommended
for LASIK dryness*
30 Single-Use Containers
0.01 fl oz (0.4 mL) each Sterile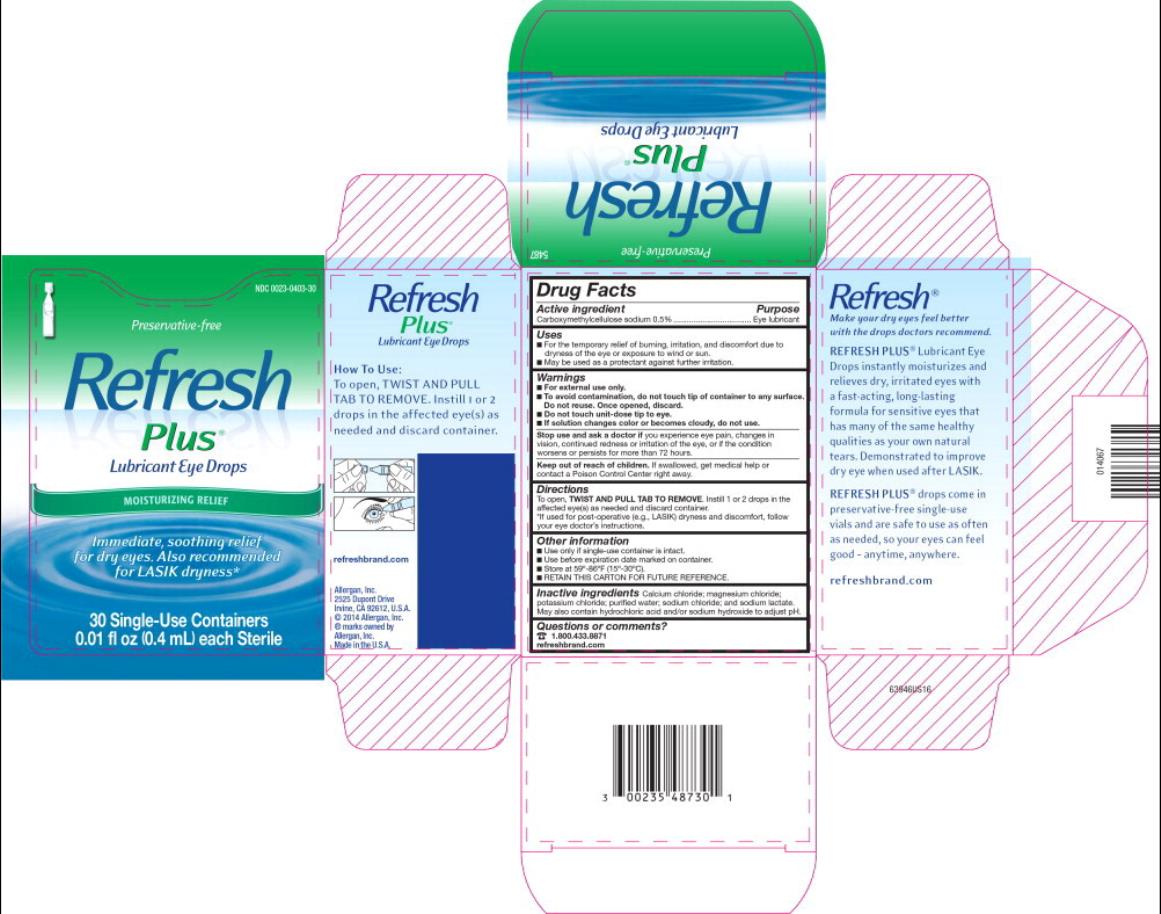
-
INGREDIENTS AND APPEARANCE
REFRESH PLUS
carboxymethylcellulose sodium solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0023-0403 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) (CARBOXYMETHYLCELLULOSE - UNII:05JZI7B19X) CARBOXYMETHYLCELLULOSE SODIUM 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-0403-05 5 in 1 CARTON 10/09/1996 1 0.4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC: 0023-0403-30 30 in 1 CARTON 10/09/1996 2 0.4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC: 0023-0403-50 50 in 1 CARTON 10/09/1996 3 0.4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 4 NDC: 0023-0403-70 70 in 1 CARTON 10/09/1996 4 0.4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 5 NDC: 0023-0403-10 100 in 1 CARTON 10/09/1996 5 0.4 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part349 10/09/1996 Labeler - Allergan, Inc. (144796497)
Trademark Results [REFRESH PLUS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REFRESH PLUS 90859576 not registered Live/Pending |
AGENTIS AIR LLC 2021-07-31 |
 REFRESH PLUS 74399667 1870318 Live/Registered |
ALLERGAN, INC. 1993-06-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.