CETIRIZINE HYDROCHLORIDE tablet, film coated
CETIRIZINE HYDROCHLORIDE by
Drug Labeling and Warnings
CETIRIZINE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by Rite Aid Corporation, Apotex Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Applicable warning(s) in 201.66(c)(5)(i) and (ii)
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults and children 6 years and over one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
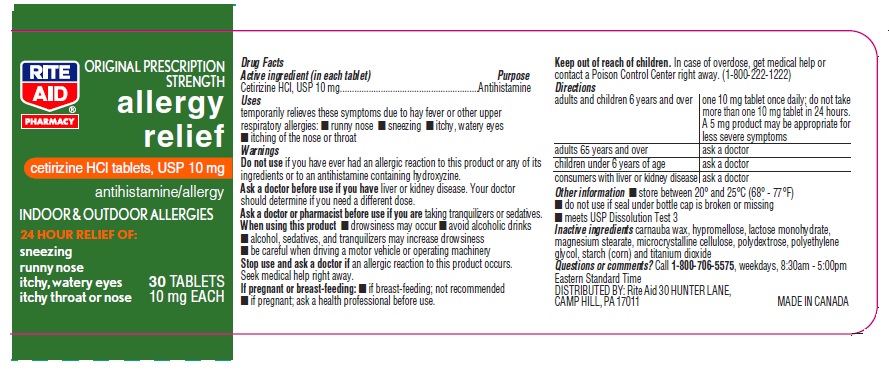
Principal Display Panel
Rite Aid NDC: 11822-7053-8
Original Prescription Strength
allergy relief
Cetirizine HCl Tablets, USP 10 mg
Antihistamine
Indoor & Outdoor Allergies
24 hour Relief of:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Throat or Nose
30 count Bottle
-
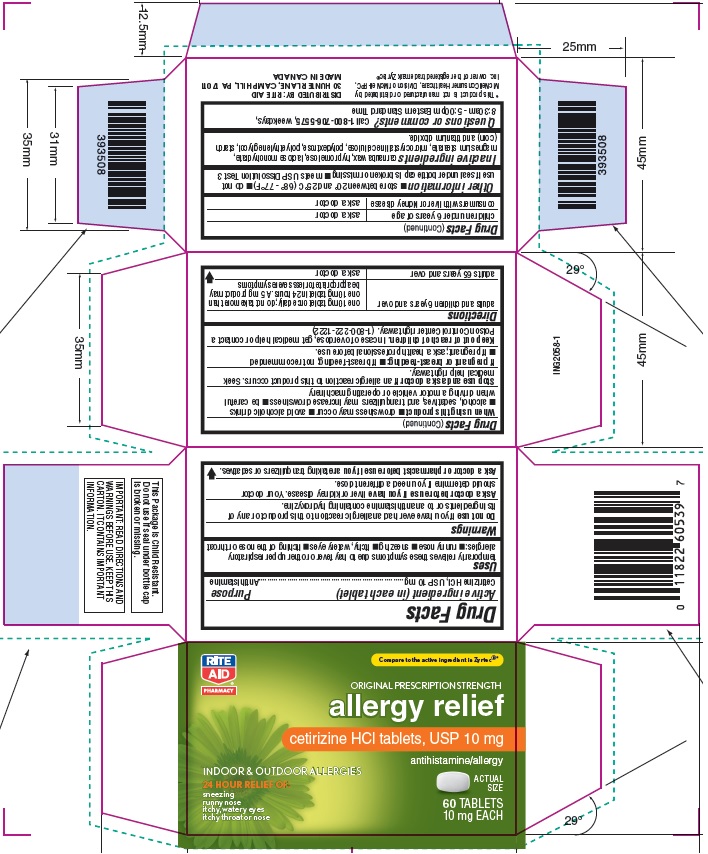
Principal Display Panel
Rite Aid NDC: 11822-6053-9
Original Prescription Strength
allergy relief
Cetirizine HCl Tablets, USP 10 mg
Antihistamine
Indoor & Outdoor Allergies
24 hour Relief of:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Throat or Nose
60 count Carton
-
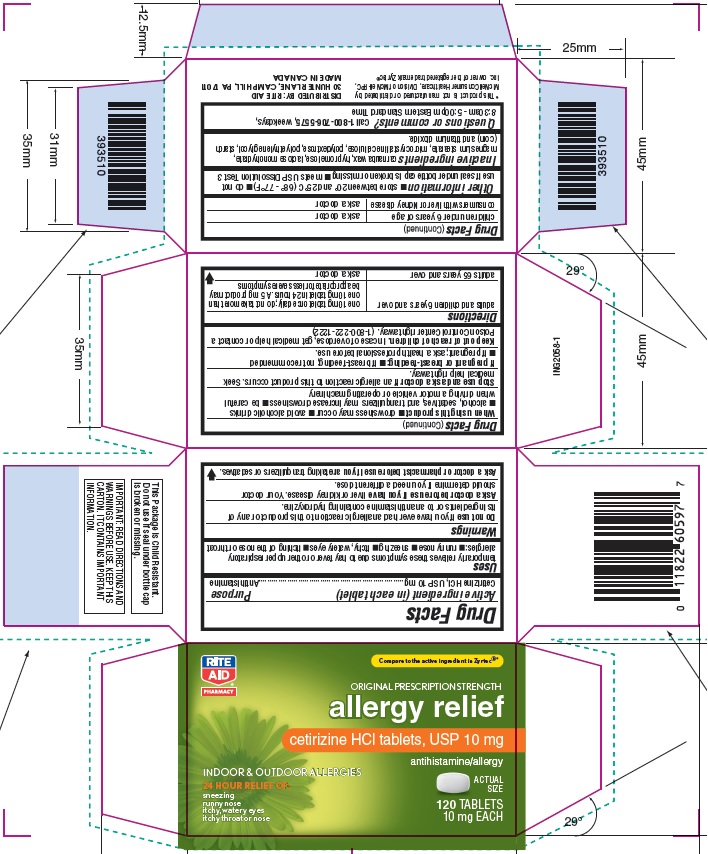
Principal Display Panel
Rite Aid NDC: 11822-6059-7
Original Prescription Strength
allergy relief
Cetirizine HCl Tablets, USP 10 mg
Antihistamine
Indoor & Outdoor Allergies
24 hour Relief of:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Throat or Nose
120 count Carton
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11822-6053 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color WHITE Score no score Shape RECTANGLE (pillow-shaped) Size 9mm Flavor Imprint Code 10MG;APO Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11822-6053-7 2 in 1 CARTON 05/25/2017 04/30/2021 1 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 11822-6053-9 1 in 1 CARTON 05/25/2017 04/30/2021 2 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078317 05/25/2017 04/30/2021 CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11822-7053 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color WHITE Score no score Shape RECTANGLE (pillow-shaped) Size 9mm Flavor Imprint Code 10MG;APO Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11822-7053-8 1 in 1 CARTON 05/25/2017 04/30/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078317 05/25/2017 04/30/2021 CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11822-6059 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color WHITE Score no score Shape RECTANGLE (pillow-shaped) Size 9mm Flavor Imprint Code 10MG;APO Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11822-6059-7 1 in 1 CARTON 05/25/2017 04/30/2021 1 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078317 05/25/2017 04/30/2021 Labeler - Rite Aid Corporation (014578892) Registrant - Apotex Inc. (209429182) Establishment Name Address ID/FEI Business Operations Apotex Inc. 209429182 analysis(11822-6053, 11822-7053, 11822-6059) , manufacture(11822-6053, 11822-7053, 11822-6059)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.