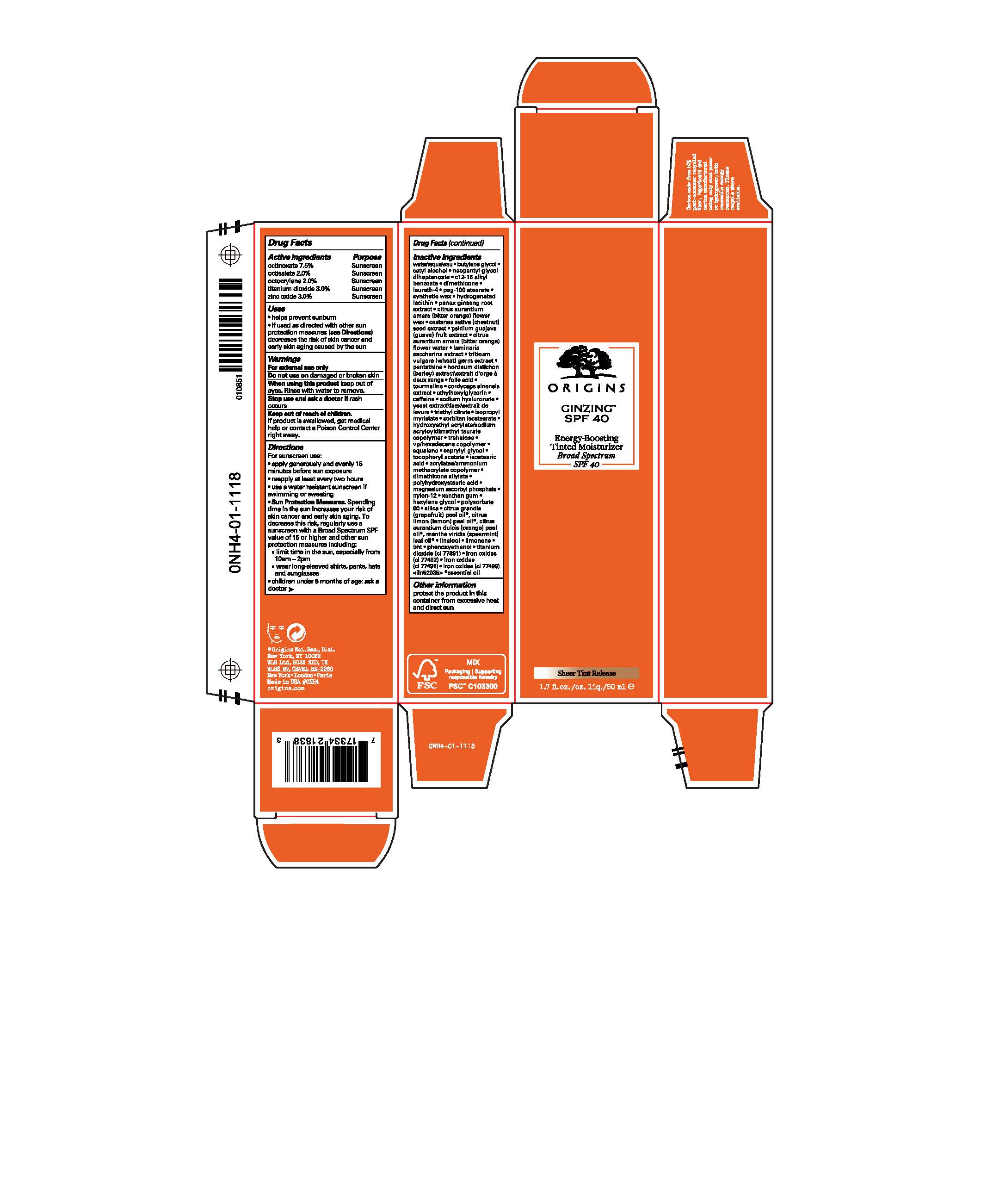
ORIGINS GINZING ENERGY-BOOSTING TINTED MOISTURIZER BROAD SPECTRUM SPF 40- octinoxate, octisalate, octocrylene, titanium dioxide, and zinc oxide liquid
ORIGINS GINZING ENERGY-BOOSTING TINTED MOISTURIZER BROAD SPECTRUM SPF 40 by
Drug Labeling and Warnings
ORIGINS GINZING ENERGY-BOOSTING TINTED MOISTURIZER BROAD SPECTRUM SPF 40 by is a Otc medication manufactured, distributed, or labeled by ORIGINS NATURAL RESOURCES INC., Estee Lauder Companies Inc., PALC, Estee Lauder Cosmetics Ltd., Whitman Laboratories Ltd., Estee Lauder N.V., The Estee Lauder Inc, Northtec LLC, PADC 1. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am – 2pm
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqualeau butylene glycol cetyl alcohol neopentyl glycol diheptanoate c12-15 alkyl benzoate dimethicone laureth-4 peg-100 stearate synthetic wax hydrogenated lecithin panax ginseng root extract citrus aurantium amara (bitter orange) flower wax castanea sativa (chestnut) seed extract psidium guajava (guava) fruit extract citrus aurantium amara (bitter orange) flower water laminaria saccharina extract triticum vulgare (wheat) germ extract pantethine hordeum distichon (barley) extract\extrait d'orge a deux rangs folic acid tourmaline cordyceps sinensis extract ethylhexylglycerin caffeine sodium hyaluronate yeast extract\faex\extrait de levure triethyl citrate isopropyl myristate sorbitan isostearate hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer trehalose vp/hexadecene copolymer squalane caprylyl glycol tocopheryl acetate isostearic acid acrylates/ammonium methacrylate copolymer dimethicone silylate polyhydroxystearic acid magnesium ascorbyl phosphate nylon-12 xanthan gum hexylene glycol polysorbate 60 silica citrus grandis (grapefruit) peel oil*, citrus limon (lemon) peel oil*, citrus aurantium dulcis (orange) peel oil*, mentha viridis (spearmint) leaf oil* linalool limonene bht phenoxyethanol titanium dioxide (ci 77891) iron oxides (ci 77492) iron oxides (ci 77491) iron oxides (ci 77499) <iln52035> *essential oil
- Other information
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
ORIGINS GINZING ENERGY-BOOSTING TINTED MOISTURIZER BROAD SPECTRUM SPF 40
octinoxate, octisalate, octocrylene, titanium dioxide, and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59427-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 20 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 30 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength BARLEY (UNII: 5PWM7YLI7R) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) AMMONIUM ACRYLOYL DIMETHYLTAURATE/METHACRYLATE, DIMETHYLACRYLAMIDE AND METHACRYLIC ACID COPOLYMER, PPG-3 GLYCERYL TRIACRYLATE CROSSLINKED (100000 MW) (UNII: WR7H9IW2XX) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL ALCOHOL (UNII: 936JST6JCN) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) LAURETH-4 (UNII: 6HQ855798J) PEG-100 STEARATE (UNII: YD01N1999R) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) SPEARMINT OIL (UNII: C3M81465G5) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+)- (UNII: F4VNO44C09) PANAX GINSENG ROOT OIL (UNII: P9T4K47OM0) CITRUS AURANTIUM FLOWER WAX (UNII: 02171IZX8X) SPANISH CHESTNUT (UNII: 2MT5XMR2YW) GUAVA (UNII: 74O70D6VG0) CITRUS AURANTIUM FLOWER OIL (UNII: D4BGE91OXH) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) WHEAT GERM (UNII: YR3G369F5A) PANTETHINE (UNII: 7K81IL792L) ALV-001 (UNII: 8ZDZ2HTA6B) FOLIC ACID (UNII: 935E97BOY8) SCHORL TOURMALINE (UNII: 173O8XLY6T) OPHIOCORDYCEPS SINENSIS (UNII: 8Q1GYP08KU) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAFFEINE (UNII: 3G6A5W338E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) TREHALOSE (UNII: B8WCK70T7I) AMMONIO METHACRYLATE COPOLYMER TYPE A (UNII: 8GQS4E66YY) SQUALANE (UNII: GW89575KF9) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) ISOSTEARIC ACID (UNII: X33R8U0062) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) NYLON-12 (UNII: 446U8J075B) XANTHAN GUM (UNII: TTV12P4NEE) HEXYLENE GLYCOL (UNII: KEH0A3F75J) POLYSORBATE 60 (UNII: CAL22UVI4M) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59427-016-01 1 in 1 CARTON 04/26/2021 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 59427-016-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 11/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/26/2021 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(59427-016) , pack(59427-016) , label(59427-016)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.