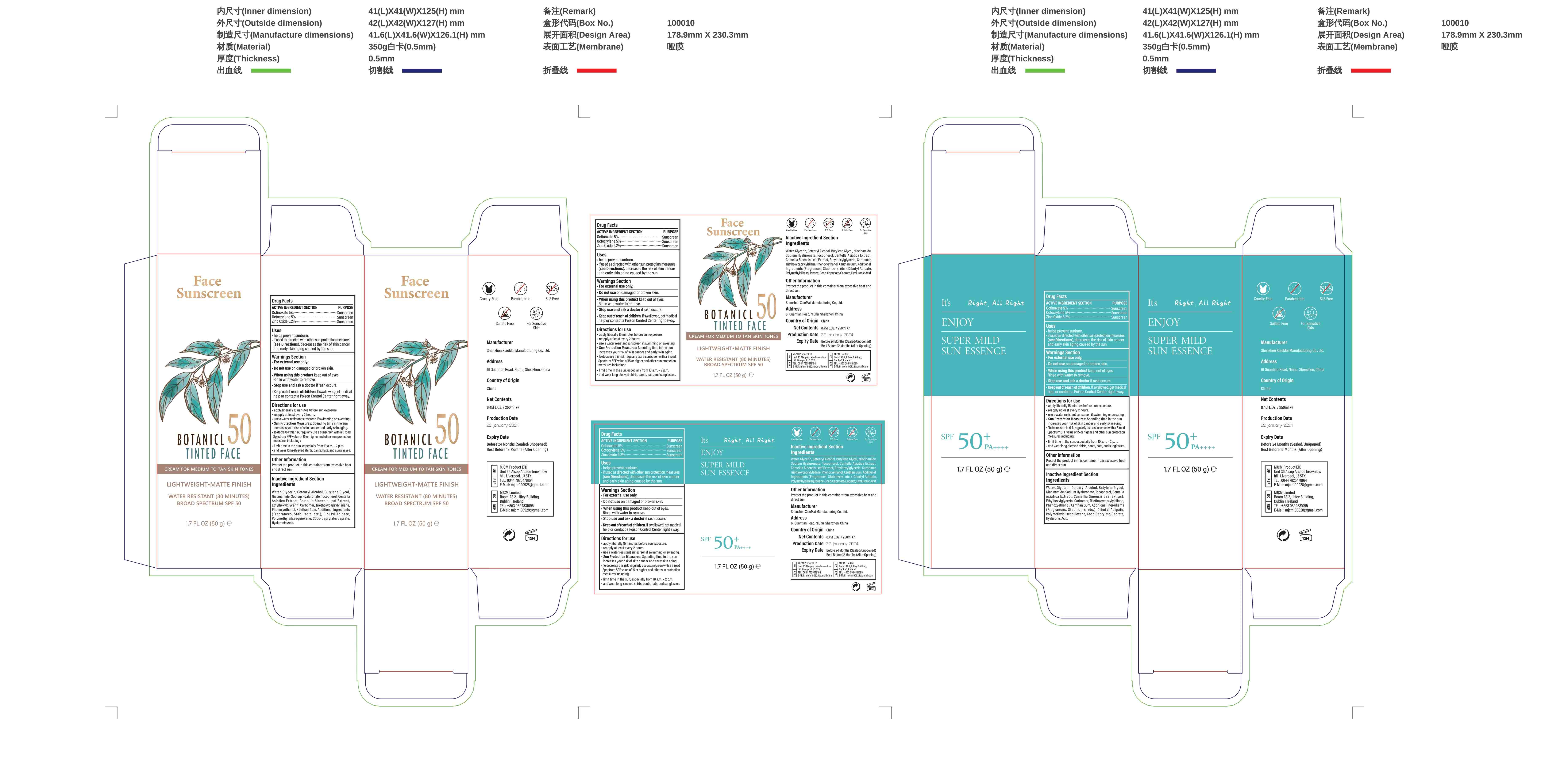
Face Sunscreen by Shenzhen Xiaomai Manufacturing Co., Ltd.
Face Sunscreen by
Drug Labeling and Warnings
Face Sunscreen by is a Otc medication manufactured, distributed, or labeled by Shenzhen Xiaomai Manufacturing Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FACE SUNSCREEN- face sunscreen cream
Shenzhen Xiaomai Manufacturing Co., Ltd.
----------
Uses
Apply sunscreen generously to exposedskin. Rub it in evenly. Reapply as neededespecially after swimming or sweating.Enjoy your sun-protected day!
Keep out of reach of children.
if swallowed, get medical help or contact a Poison Control Center right away.
Directions
Directions for use
-apply liberally 15 minutes before sun exposure.
-reapply at least every 2 hours.
-use a water resistant sunscreen if swimming or sweating.
-Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging
-To decrease this risk, regularly use a sunscreen with a B road.
-Spectrum SPF value of15 or higher and other sun protection:
-limit time in the sun, especially from 10 a.m.-2 p.m.
-and wear long-sleeved shirts, pants, hats, and sunglasses
Other information
Before use, read all information on thecarton Store at controlled room temperature20 to 25 C (68 to 77°F)
Inactive ingredients
Water, Glycerin, Cetearyl Alcohol, Butylene Glycol,Niacinamide, Sodium Hyaluronate, Tocopherol, Centella,Asiatica Extract,Camellia Sinensis Leaf Extract,Ethylhexylglycerin, Carbomer, Triethoxycaprylyisilane,Phenoxyethanol,Xanthan Gum,Additional Ingredients(Fragrances,Stabilizers,etc.),Dibutyl Adipate,Polymethylsiisesquioxane,Coco-Caprylate/Caprate,Hyaluronic Acid.
| FACE SUNSCREEN
face sunscreen cream |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Shenzhen Xiaomai Manufacturing Co., Ltd. (712999147) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Xiaomai Manufacturing Co., Ltd. | 712999147 | manufacture(83872-013) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.