CHILDRENS BENADRYL DYE-FREE ALLERGY- diphenhydramine hydrochloride solution
Childrens Benadryl DYE-FREE ALLERGY by
Drug Labeling and Warnings
Childrens Benadryl DYE-FREE ALLERGY by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
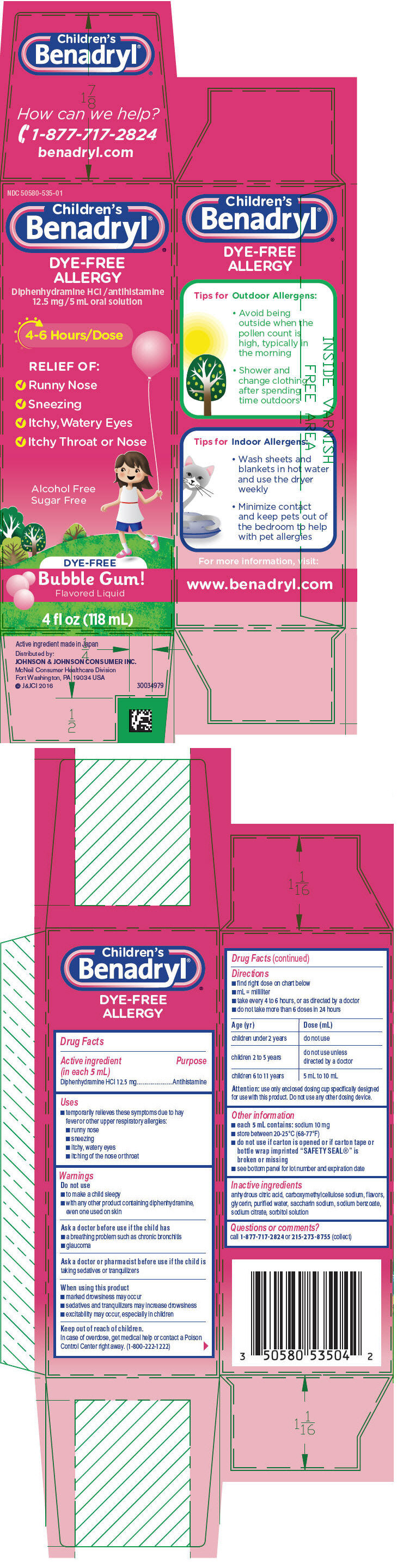
- Active ingredient (in each 5 mL)
- Purpose
- Uses
- Warnings
-
Directions
- find right dose on chart below
- mL = milliliter
- take every 4 to 6 hours, or as directed by a doctor
- do not takemore than 6 doses in 24 hours
Age (yr) Dose (mL) children under 2 years do not use children 2 to 5 years do not use unless directed by a doctor children 6 to 11 years 5 mL to 10 mL Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
NDC: 50580-535-01
Children's
Benadryl®DYE-FREE
ALLERGYDiphenhydramine HCl /antihistamine
12.5mg/5mL oral solution4-6 Hours/Dose
RELIEF OF:
- ✔ Runny Nose
- ✔ Sneezing
- ✔ Itchy,Watery Eyes
- ✔ Itchy Throat or Nose
Alcohol Free
Sugar FreeDYE-FREE
Bubble Gum!
Flavored Liquid4 fl oz (118mL)
-
INGREDIENTS AND APPEARANCE
CHILDRENS BENADRYL DYE-FREE ALLERGY
diphenhydramine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50580-535 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diphenhydramine hydrochloride (UNII: TC2D6JAD40) (diphenhydramine - UNII:8GTS82S83M) Diphenhydramine hydrochloride 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) carboxymethylcellulose sodium, unspecified form (UNII: K679OBS311) glycerin (UNII: PDC6A3C0OX) water (UNII: 059QF0KO0R) saccharin sodium (UNII: SB8ZUX40TY) sodium benzoate (UNII: OJ245FE5EU) sodium citrate, unspecified form (UNII: 1Q73Q2JULR) sorbitol (UNII: 506T60A25R) Product Characteristics Color WHITE (Clear, colorless) Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50580-535-01 1 in 1 CARTON 12/01/2015 1 118 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 07/01/2008 Labeler - Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division (878046358)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.