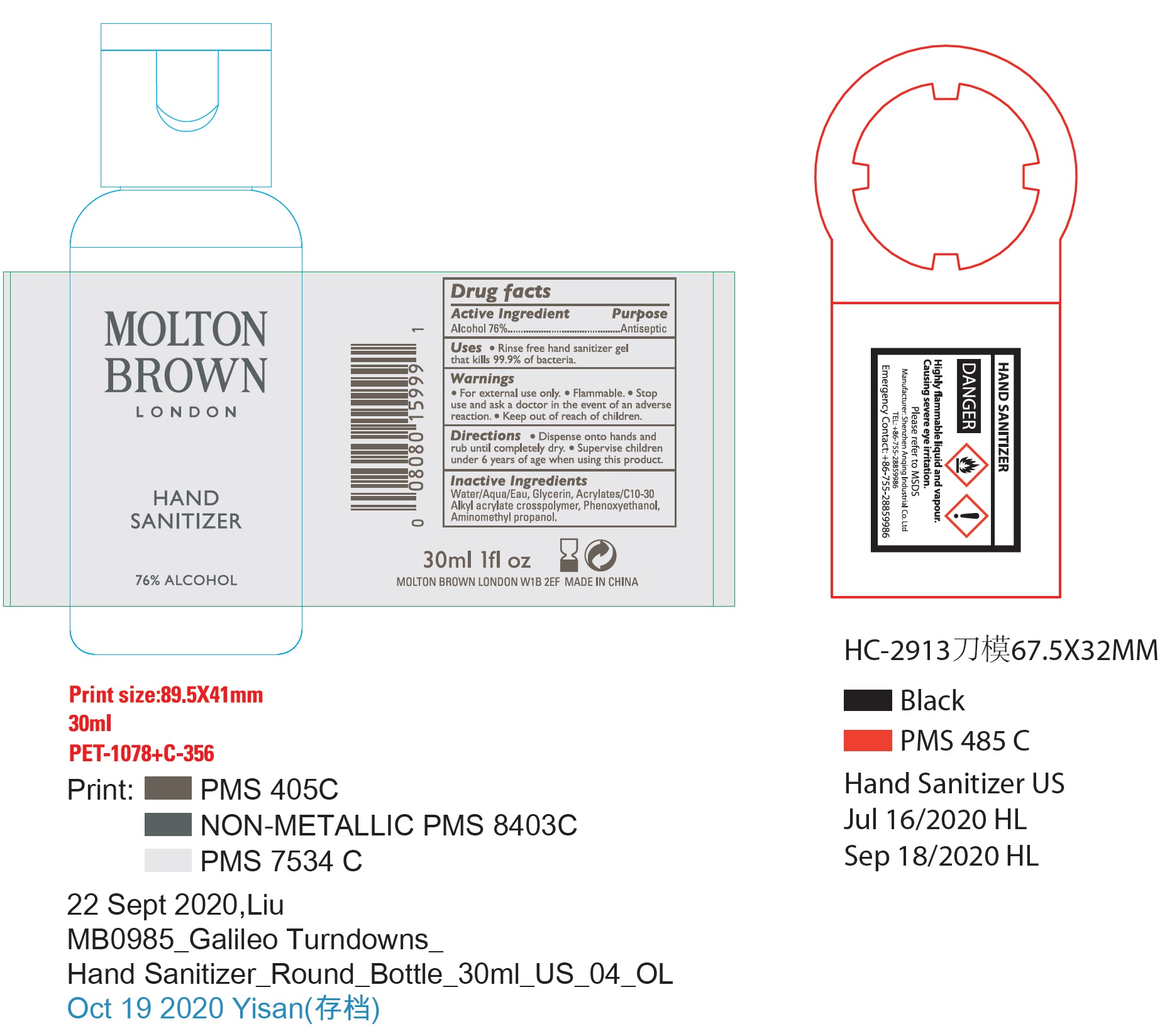
Molton Brown Hand Sanitizer
Molton Brown Hand Sanitizer by
Drug Labeling and Warnings
Molton Brown Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Molton Brown Ltd., Shenzhen Anqing Industrial Co. Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MOLTON BROWN HAND SANITIZER- alcohol gel
Molton Brown Ltd.
----------
Molton Brown Hand Sanitizer
Directions
- Dispense onto hands and rub until completely dry.
- Supervise children under 6 years of age when using this product.
| MOLTON BROWN HAND SANITIZER
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Molton Brown Ltd. (769334822) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Anqing Industrial Co. Ltd | 554541175 | manufacture(68814-010) | |
Revised: 3/2024
Document Id: 14956bbc-fc43-83a9-e063-6394a90a0e7b
Set id: 10dc1613-acf4-4f65-b709-8f3d8a8b136c
Version: 3
Effective Time: 20240326
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.