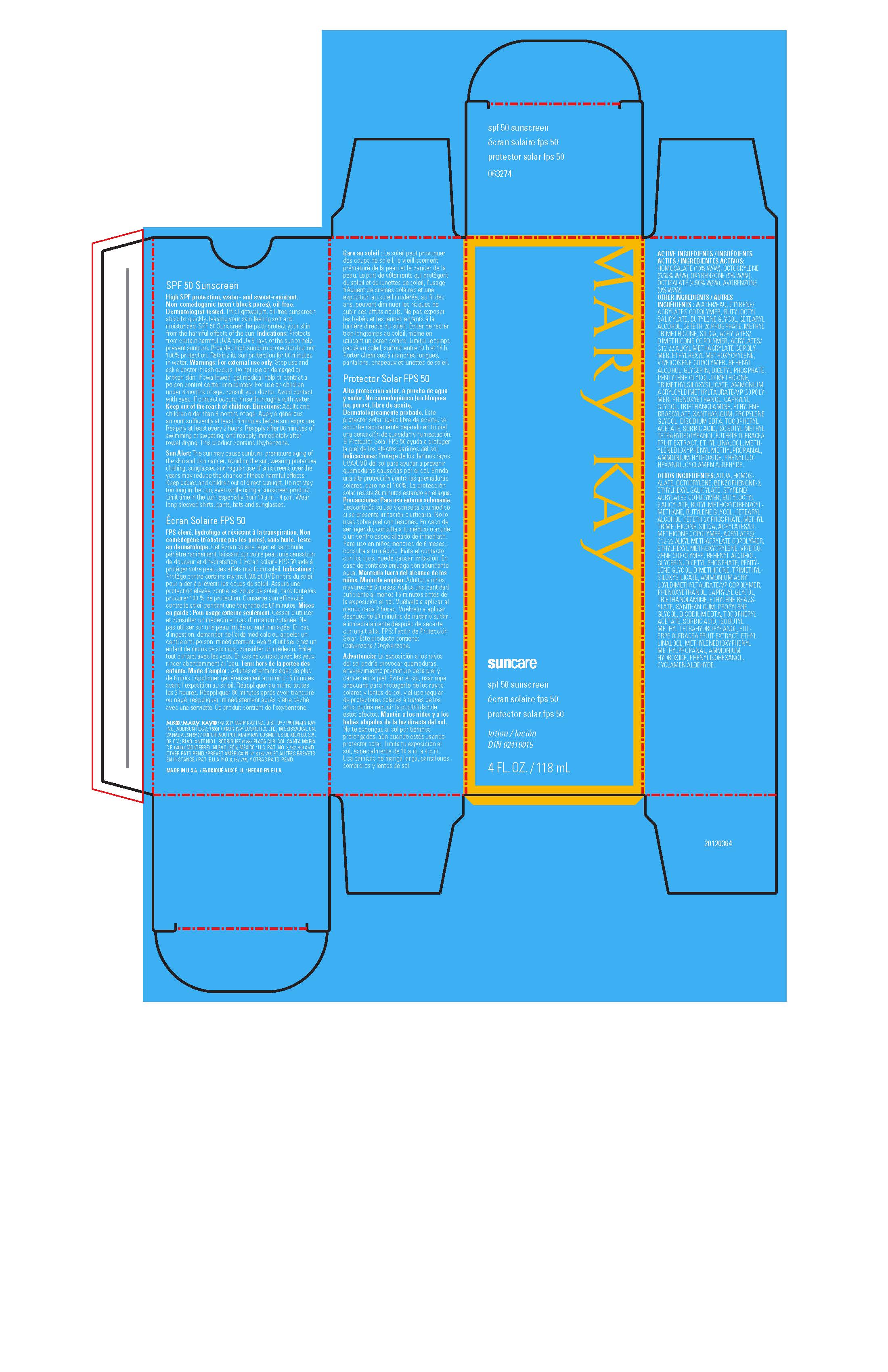
MARY KAY SUNCARE SPF 50 SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotion
Mary Kay Suncare SPF 50 Sunscreen by
Drug Labeling and Warnings
Mary Kay Suncare SPF 50 Sunscreen by is a Otc medication manufactured, distributed, or labeled by Mary Kay Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENTS:
- Indications:
-
Warnings:
For external use only.
Stop use and ask a doctor if rash occurs.
Do not use on damaged or broken skin.
If swallowed, get medical help or contact a poison control center immediately.
For use on children under 6 months of age, consult your doctor.
Avoid contact with eyes.
If contact occurs, rinse thoroughly with water.
Keep out of reach of children.
Sun Alert: The sun may cause sunburn, premature aging of the skin and skin cancer.
Avoiding the sun, wearing protective clothing, sunglasses, and regular use of sunscreens over the years may reduce the chance of these harmful effects.
Keep babies and children out of direct sunlight.
Do not stay too long in the sun, even while using a sunscreen product.
Limit time in the sun, especially from 10 a.m. - 4 p.m.).
Wear long-sleeved shirts, pants, hats and sunglasses.
This product contains Oxybenzone.
- Directions:
-
Ingredients:
WATER/EAU, STYRENE/ACRYLATES COPOLYMER, BUTYLOCTYL SALICYLATE,
BUTYLENE GLYCOL, CETEARYL ALCOHOL, CETETH-20 PHOSPHATE, METHYL TRIMETHICONE,
SILICA, ACRYLATES/DIMETHICONE COPOLYMER, ACRYLATES/C12-22 ALKYL METHACRYLATE COPOLYMER,
ETHYLHEXYL METHOXYCRYLENE, VP/EICOSENE COPOLYMER, BEHENYL ALCOHOL, GLYCERIN,
DICETYL PHOSPHATE, PENTYLENE GLYCOL, DIMETHICONE, TRIMETHYLSILOXYSILICATE,
AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, PHENOXYETHANOL, CAPRYLYL GLYCOL,
TRIETHANOLAMINE, ETHYLENE BRASSYLATE, XANTHAN GUM, PROPYLENE GLYCOL, DISODIUM EDTA,
TOCOPHERYL ACETATE, SORBIC ACID, ISOBUTYL METHYL TETRAHYDROPYRANOL,
EUTERPE OLERACEA FRUIT EXTRACT, ETHYL LINALOOL, METHYLENEDIOXYPHENYL METHYLPROPANAL,
AMMONIUM HYDROXIDE, PHENYLISOHEXANOL, CYCLAMEN ALDEHYDE.
- Principal Display Panel - 118 ml carton
-
INGREDIENTS AND APPEARANCE
MARY KAY SUNCARE SPF 50 SUNSCREEN
avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51531-3274 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5.5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETETH-20 PHOSPHATE (UNII: 921FTA1500) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DOCOSANOL (UNII: 9G1OE216XY) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) GLYCERIN (UNII: PDC6A3C0OX) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) PENTYLENE GLYCOL (UNII: 50C1307PZG) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) DIMETHICONE (UNII: 92RU3N3Y1O) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TROLAMINE (UNII: 9O3K93S3TK) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SORBIC ACID (UNII: X045WJ989B) 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) 3-(3,4-METHYLENEDIOXYPHENYL)-2-METHYLPROPANAL (UNII: L65EG8H6PA) ACAI (UNII: 46AM2VJ0AW) ETHYL LINALOOL (UNII: SF2JS9GF5T) AMMONIA (UNII: 5138Q19F1X) PHENYLISOHEXANOL (UNII: M56178H183) CYCLAMEN ALDEHYDE (UNII: 4U37UX0E1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51531-3274-4 1 in 1 CARTON 12/16/2013 1 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 12/16/2013 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-3274) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 081158134 manufacture(51531-3274)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.