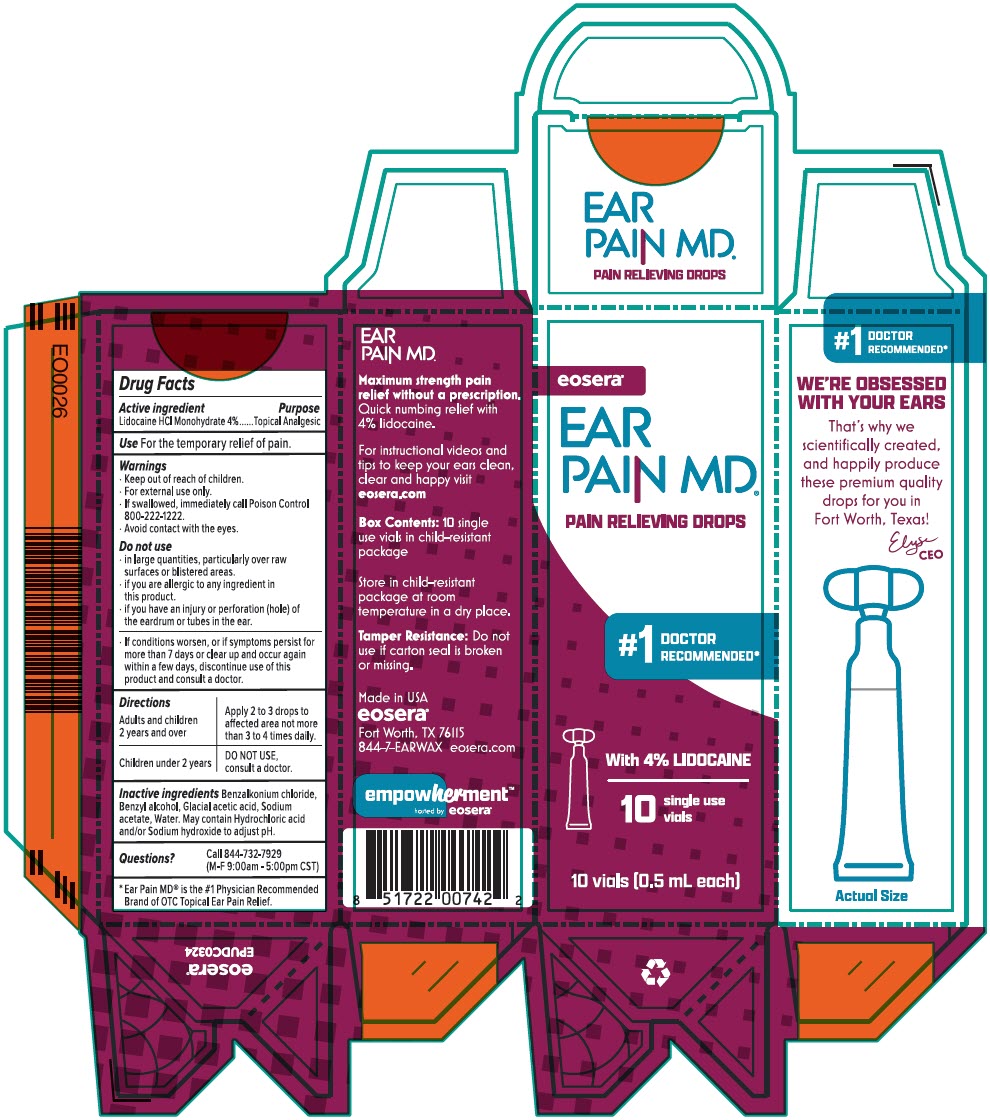
Ear Pain MD Unit Dose by Eosera, Inc Ear Pain MD® Unit Dose
Ear Pain MD Unit Dose by
Drug Labeling and Warnings
Ear Pain MD Unit Dose by is a Otc medication manufactured, distributed, or labeled by Eosera, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EAR PAIN MD UNIT DOSE- lidocaine hydrochloride liquidÂ
Eosera Inc
----------
Ear Pain MD® Unit Dose
Warnings
- For external use only.
- If swallowed, immediately call Poison Control 800-222-1222
- Avoid contact with the eyes.
Do not use
- in large quantities, particularly over raw skin surfaces or blistered areas.
- if you are allergic to any ingredient in this product.
- if you have an injury or perforation (hole) of the eardrum, including tubes in the ear.
- If conditions worsen, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
Directions
| Adults and children 2 years of age and older | Apply 2 to 3 drops to the affected area not more than 3 to 4 times daily. |
| For children under 2 | DO NOT USE, consult a doctor. |
| EAR PAIN MD UNIT DOSEÂ
lidocaine hydrochloride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler -Â Eosera Inc (079789050) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eosera Inc | 079789050 | MANUFACTURE(72429-0003) | |
Revised: 8/2024
Â
Document Id: ebb7d481-2404-4b77-8d19-d9b2a9e8804f
Set id: 10fef6ba-297f-4e5f-8769-d12ba94cdcba
Version: 2
Effective Time: 20240821
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.