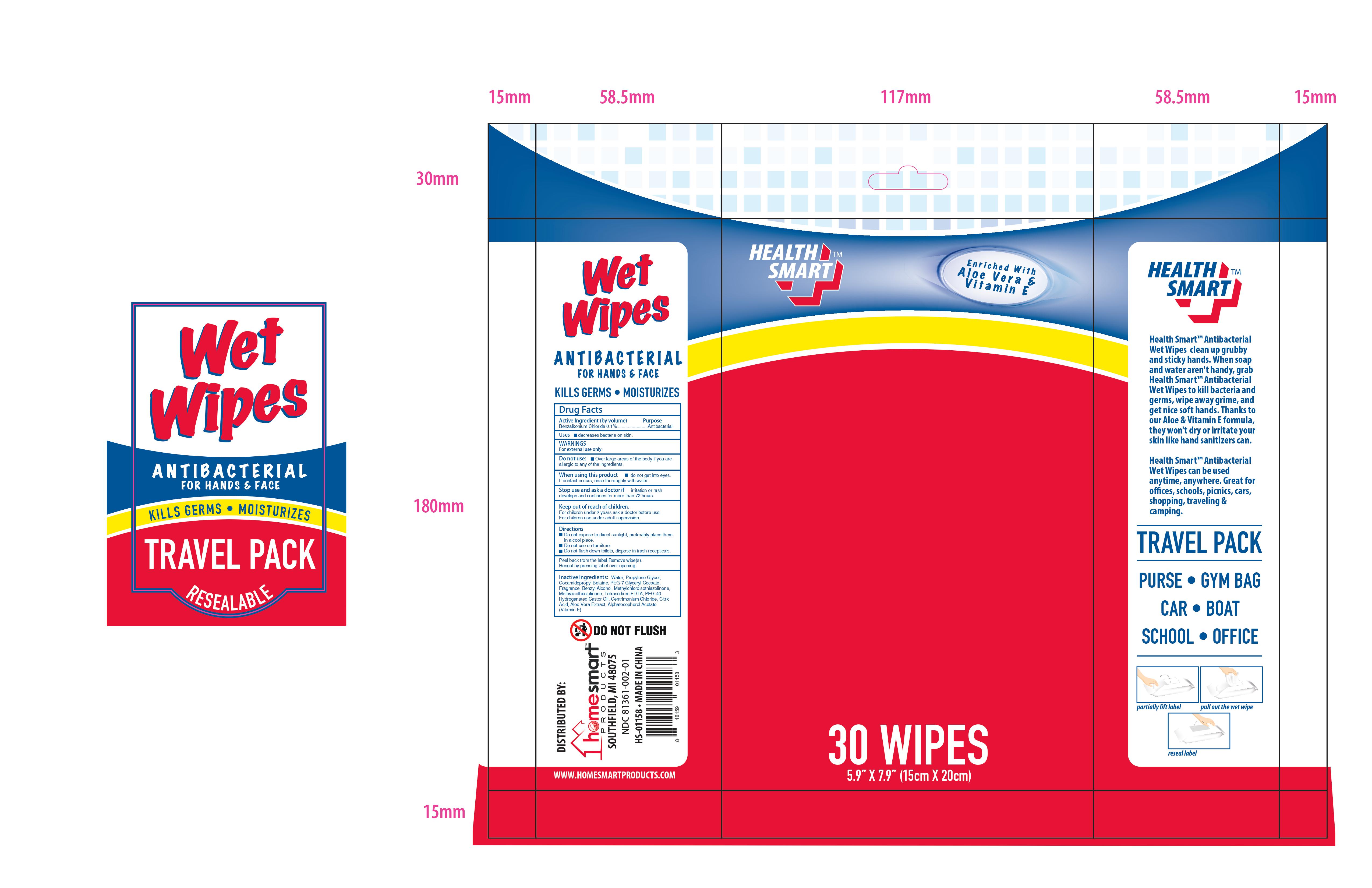
WET WIPES ANTIBACTERIAL TRAVEL
WET WIPES ANTIBACTERIAL TRAVEL by
Drug Labeling and Warnings
WET WIPES ANTIBACTERIAL TRAVEL by is a Otc medication manufactured, distributed, or labeled by International Wholesale, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WET WIPES ANTIBACTERIAL TRAVEL- benzalkonium chloride cloth
International Wholesale, Inc.
----------
WET WIPES ANTIBACTERIAL TRAVEL
Directions
Do not expose to direct sunlight, preferably place them in a cool place. Do not use on furniture. Do not flush down toilets, dispose in trash recepticals.
Remove lid and open the seal. Pull up the corner of the center sheet, twist it and thread through the dispenser split in the lid. Pull sheet out at an angle. When finished close lid flap to retain moisture.
Inactive Ingredients:
Water, Propylene Glycol, Cocamidopropyl Betaine, PEG-7 Glyceryl Cocoate, Fragrance, Benzyl Alcohol, Methylchloroisothiazolinone, Methylisothiazolinone, Tetrasodium EDTA, PEG-40 Hydrogenated Castor Oil, Cetrimonium Chloride, Citric Acid, Aloe Vera Extract, Alphatocopherol, Acetate (Vitamin E)
| WET WIPES ANTIBACTERIAL TRAVEL
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - International Wholesale, Inc. (161872676) |