VAXNEUVANCE- pneumococcal 15-valent conjugate vaccine crm197 protein adsorbed injection, suspension
VAXNEUVANCE by
Drug Labeling and Warnings
VAXNEUVANCE by is a Other medication manufactured, distributed, or labeled by Merck Sharp & Dohme LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VAXNEUVANCE safely and effectively. See full prescribing information for VAXNEUVANCE.
VAXNEUVANCE® (Pneumococcal 15-valent Conjugate Vaccine)
Suspension for Intramuscular Injection
Initial U.S. Approval: 2021INDICATIONS AND USAGE
VAXNEUVANCE® is a vaccine indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F in individuals 6 weeks of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Suspension for injection (0.5 mL dose), supplied as a single-dose prefilled syringe. (3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid. (4)
WARNINGS AND PRECAUTIONS
Apnea following intramuscular vaccination has been observed in some infants born prematurely. A decision about when to administer VAXNEUVANCE to infants born prematurely should be based on consideration of the individual infant’s medical status and the potential benefits and possible risks of vaccination. (5.3)
ADVERSE REACTIONS
The most commonly reported solicited adverse reactions:
- in children vaccinated with a 4-dose series at 2, 4, 6 and 12 through 15 months of age, provided as a range across the series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%). (6.1)
- in children and adolescents 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%). (6.1)
- in adults 18 through 49 years of age were: injection-site pain (75.8%), fatigue (34.3%), myalgia (28.8%), headache (26.5%), injection-site swelling (21.7%), injection-site erythema (15.1%) and arthralgia (12.7%). (6.1)
- in adults 50 years of age and older were: injection-site pain (66.8%), myalgia (26.9%), fatigue (21.5%), headache (18.9%), injection-site swelling (15.4%), injection-site erythema (10.9%) and arthralgia (7.7%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
2.3 Vaccination Schedule
2.4 Catch-Up Vaccination Schedule in Children and Adolescents
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
5.2 Altered Immunocompetence
5.3 Apnea in Premature Infants
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Individuals at Increased Risk for Pneumococcal Disease
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Trials in Children
14.2 Clinical Trials in Pneumococcal Vaccine-Naïve Adults
14.3 Concomitant Vaccination
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.2 Administration
Hold the prefilled syringe horizontally and shake vigorously immediately prior to use to obtain an opalescent suspension. Do not use the vaccine if it cannot be resuspended. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
2.3 Vaccination Schedule
Children
Administer VAXNEUVANCE as a 4-dose series at 2, 4, 6 and 12 through 15 months of age (and at least 2 months after the third dose). The first dose may be given as early as 6 weeks of age.
The 4-dose series initiated with a lower valency pneumococcal conjugate vaccine can be completed with VAXNEUVANCE [see Clinical Studies (14.1)].
Adults
Administer VAXNEUVANCE as a single dose in adults 18 years of age and older.
2.4 Catch-Up Vaccination Schedule in Children and Adolescents
Children 7 months through 17 years of age who have never received a pneumococcal conjugate vaccine may receive VAXNEUVANCE according to the schedule in Table 1:
Table 1: Catch-Up Vaccination Schedule for Individuals Initiating Vaccination at 7 Months Through 17 Years of Age Age at First Dose Total Number of 0.5 mL Doses - * The first 2 doses are given at least 4 weeks apart; third dose given after the one-year birthday, separated from the second dose by at least 2 months.
- † Two doses at least 2 months apart.
7 through 11 months of age 3* 12 through 23 months of age 2† 2 years through 17 years of age 1 Children and Adolescents Previously Vaccinated with a Pneumococcal Conjugate Vaccine
Administer a single dose of VAXNEUVANCE to children and adolescents 2 years through 17 years of age who have received an incomplete series of another pneumococcal conjugate vaccine. At least 2 months should elapse between receipt of the last dose of another pneumococcal conjugate vaccine and administration of VAXNEUVANCE.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer VAXNEUVANCE to individuals with a severe allergic reaction (e.g., anaphylaxis) to any component of VAXNEUVANCE or to diphtheria toxoid. [See Description (11).]
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
Appropriate medical treatment to manage allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of VAXNEUVANCE.
5.2 Altered Immunocompetence
Some individuals with altered immunocompetence, including those receiving immunosuppressive therapy, may have a reduced immune response to VAXNEUVANCE. [See Use in Specific Populations (8.6).]
5.3 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. A decision about when to administer VAXNEUVANCE to infants born prematurely should be based on consideration of the individual infant’s medical status and the potential benefits and possible risks of vaccination.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The most commonly reported solicited adverse reactions in children vaccinated with a 4-dose series at 2, 4, 6, and 12 through 15 months of age, provided as a range across the series, were: irritability (57.3% to 63.4%), somnolence (24.2% to 47.5%), injection-site pain (25.9% to 40.3%), fever ≥38.0°C (13.3% to 20.4%), decreased appetite (14.1% to 19.0%), injection-site induration (13.2% to 15.4%), injection-site erythema (13.7% to 21.4%) and injection-site swelling (11.3% to 13.4%).
The most commonly reported solicited adverse reactions in children and adolescents 2 through 17 years of age vaccinated with a single dose were: injection-site pain (54.8%), myalgia (23.7%), injection-site swelling (20.9%), injection-site erythema (19.2%), fatigue (15.8%), headache (11.9%) and injection-site induration (6.8%).
The most commonly reported solicited adverse reactions in adults 18 through 49 years of age were: injection-site pain (75.8%), fatigue (34.3%), myalgia (28.8%), headache (26.5%), injection-site swelling (21.7%), injection-site erythema (15.1%) and arthralgia (12.7%).
The most commonly reported solicited adverse reactions in adults 50 years of age and older were: injection-site pain (66.8%), myalgia (26.9%), fatigue (21.5%), headache (18.9%), injection-site swelling (15.4%), injection-site erythema (10.9%) and arthralgia (7.7%).
Clinical Trials Experience in Children 6 Weeks Through 17 Years of Age
Safety Assessment in Children Receiving a 4-Dose Series
The safety of VAXNEUVANCE in healthy infants (6 weeks through 11 months of age) and children (12 months through 15 months of age) was assessed in 4 randomized, double-blind clinical studies (Studies 8-11 (NCT03893448, NCT03620162, NCT03692871 and NCT02987972)) conducted in the Americas, Europe, and Asia Pacific. These studies included 3,349 participants who received at least one dose of a 4-dose series of VAXNEUVANCE, 1,814 participants who received at least one dose of a 4-dose series of Prevnar 13 [Pneumococcal 13-valent Conjugate Vaccine (Diphtheria CRM197 Protein)], and 538 participants who received VAXNEUVANCE to complete a 4-dose series of pneumococcal conjugate vaccine initiated with Prevnar 13. In the United States (including Puerto Rico), 2,827 participants received at least one dose of either VAXNEUVANCE or Prevnar 13 and 2,409 participants completed a 4-dose series of either vaccine. Overall, the median age of the participants was 9.0 weeks (6-12 weeks) and 48.6% were female. The racial distribution was as follows: 57.1% were White, 26.4% were Asian, 9.5% were Multi-racial, 4.7% were Black or African American, and 18.8% were of Hispanic or Latino ethnicity. There were no meaningful differences in demographic characteristics across the vaccination groups.
In Studies 8 and 9, Pentacel (Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate [Tetanus Toxoid Conjugate] Vaccine) (DTaP-IPV-Hib vaccine) for US participants or a non-US-licensed DTaP-IPV-Hib vaccine for non-US participants was administered concomitantly with VAXNEUVANCE at 2, 4 and 6 months of age. RotaTeq (Rotavirus Vaccine, Live, Oral, Pentavalent) and RECOMBIVAX HB (Hepatitis B Vaccine [Recombinant]) were also administered concomitantly at 2, 4, and 6 months of age. M-M-R II (Measles, Mumps, and Rubella Virus Vaccine Live), VAQTA (Hepatitis A Vaccine, Inactivated), VARIVAX (Varicella Virus Vaccine Live), and Hiberix (Haemophilus b Conjugate Vaccine [Tetanus Toxoid Conjugate]) were administered concomitantly with VAXNEUVANCE at 12 through 15 months of age. Study 9 also evaluated the use of VAXNEUVANCE to complete a pneumococcal conjugate vaccine series initiated with Prevnar 13.
Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination. Study investigators reviewed the VRC with the participant or participant’s legal guardian 15 days postvaccination to ensure consistency with protocol definitions. The analyses presented in Tables 2-3 below reflect the information based on the final assessment by the study investigators. Injection-site adverse events and systemic adverse events were solicited on Day 1 through Day 14 postvaccination. Body temperature was solicited on Day 1 through Day 7 postvaccination via rectal or axillary measurement. Unsolicited adverse events were monitored using the VRC through 14 days postvaccination. The duration of the safety follow-up period for serious adverse events following the last study vaccination was 1 month in Study 11 and 6 months in Studies 8-10.
Solicited Adverse Reactions in Children Receiving a 4-Dose Series
Study 8 was a multicenter, double-blind, active comparator-controlled study that assessed the safety of VAXNEUVANCE when administered as a 4-dose series in children (N=858 received VAXNEUVANCE and N=855 received Prevnar 13). The percentage of US participants with solicited adverse reactions that occurred within 14 days following administration of VAXNEUVANCE or Prevnar 13 are shown in Tables 2-3. Solicited adverse reactions following administration of VAXNEUVANCE lasted a median of 1 day with 90.6% of reactions lasting ≤3 days.
Table 2: Percentage of US Participants with Solicited Local Adverse Reactions in Infants at 2, 4, 6 and 12 through 15 Months of Age After Vaccination (Study 8)* Dose Dose 1 Dose 2 Dose 3 Dose 4 VAXNEUVANCE (%)
N=598Prevnar 13 (%)
N=600VAXNEUVANCE (%)
N=584Prevnar 13 (%)
N=570VAXNEUVANCE (%)
N=559Prevnar 13 (%)
N=540VAXNEUVANCE (%)
N=532Prevnar 13 (%)
N=507N=Number of participants vaccinated, including those with missing solicited adverse event data. The percentage of participants with missing solicited adverse event data, provided as a range across the 4-dose series, was 0.8% to 3.9%. - * Study 8 (NCT03893448) was a randomized, double-blind, active comparator-controlled clinical study. Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination following each dose. The table represents the final assessment by the study investigators upon review of the VRC 15 days postvaccination, to ensure consistency with protocol definitions.
- † Solicited on Day 1 through Day 14 postvaccination following each dose.
- ‡ Mild: awareness of symptoms, but easily tolerated; moderate: definitely acting like something is wrong; severe: extremely distressed or unable to do usual activities.
Local Reactions† Pain‡ Any 40.3 39.5 32.0 28.8 30.8 26.9 25.9 25.0 Mild 24.1 23.2 18.7 14.7 17.9 16.7 16.9 16.4 Moderate 14.7 15.2 12.5 13.3 12.3 10.0 8.8 8.7 Severe 1.5 1.2 0.9 0.7 0.5 0.2 0.2 0.0 Induration Any 14.0 12.7 13.2 16.1 15.4 16.3 13.7 14.6 ≤2.5 cm 11.0 10.0 9.1 11.4 10.7 11.5 7.5 8.5 2.6-7.6 cm 2.8 5.4 4.1 4.7 4.7 4.8 6.2 6.1 >7.6 cm 0.0 0.2 0.0 0.0 0.0 0.0 0.0 0.0 Erythema Any 13.7 14.7 16.4 22.5 20.4 23.9 21.4 24.1 ≤2.5 cm 11.0 10.8 12.7 16.7 15.4 17.4 14.7 16.8 2.6-7.6 cm 2.3 3.5 3.8 5.6 4.8 6.5 6.8 7.1 >7.6 cm 0.3 0.2 0.0 0.2 0.2 0.0 0.0 0.2 Swelling Any 12.9 12.7 13.2 11.4 13.4 10.4 11.3 10.8 ≤2.5 cm 9.5 7.2 8.2 6.5 8.6 5.7 5.8 7.3 2.6-7.6 cm 3.2 5.3 4.8 4.6 4.8 4.4 5.5 3.4 >7.6 cm 0.0 0.2 0.2 0.0 0.0 0.0 0.0 0.0 Table 3: Percentage of US Participants with Solicited Systemic Adverse Reactions in Infants at 2, 4, 6 and 12 through 15 Months of Age After Vaccination (Study 8)* Dose Dose 1 Dose 2 Dose 3 Dose 4 VAXNEUVANCE (%)
N=598Prevnar 13 (%)
N=600VAXNEUVANCE (%)
N=584Prevnar 13 (%)
N=570VAXNEUVANCE (%)
N=559Prevnar 13 (%)
N=540VAXNEUVANCE (%)
N=532Prevnar 13 (%)
N=507Following Doses 1-3, rectal temperature measurements were provided for 76.7% to 77.6% of participants and axillary temperature measurements were provided for 22.4% to 23.3% of participants, provided as a range across the doses.
Following Dose 4, rectal temperature measurements were provided for 70.6% of participants and axillary temperature measurements were provided for 29.4% of participants.
N=Number of participants vaccinated, including those with missing solicited adverse event data. The percentage of participants with missing solicited adverse event data, provided as a range across the 4-dose series, was 0.8% to 3.9%.- * Study 8 (NCT03893448) was a randomized, double-blind, active comparator-controlled clinical study. Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination following each dose. The table represents the final assessment by the study investigators upon review of the VRC 15 days postvaccination, to ensure consistency with protocol definitions.
- † Solicited on Day 1 through Day 14 postvaccination following each dose.
- ‡ Mild: awareness of symptoms, but easily tolerated; moderate: definitely acting like something is wrong; severe: extremely distressed or unable to do usual activities.
- § Solicited on Day 1 through Day 7 postvaccination following each dose.
- ¶ Percentages reflect the number of participants with temperature data.
Systemic Reactions† Irritability‡ Any 63.4 67.3 57.4 58.1 59.0 55.4 57.3 56.6 Mild 27.3 29.3 23.6 21.9 30.2 28.9 28.0 26.6 Moderate 31.4 33.0 30.0 33.2 25.0 24.4 26.7 27.4 Severe 4.7 5.0 3.6 3.0 3.8 2.0 2.6 2.6 Somnolence‡ Any 47.5 52.7 35.6 39.3 31.1 30.2 24.2 29.6 Mild 24.2 29.5 20.2 18.8 19.1 16.3 13.9 17.0 Moderate 21.6 21.8 14.6 19.6 11.4 12.8 10.0 11.8 Severe 1.7 1.3 0.9 0.9 0.5 1.1 0.4 0.8 Decreased appetite‡ Any 18.2 19.0 19.0 16.0 14.1 17.8 17.5 16.4 Mild 11.0 11.2 12.0 8.2 7.5 11.1 9.2 10.7 Moderate 6.7 7.2 7.0 7.4 6.3 6.5 7.9 5.5 Severe 0.5 0.7 0.0 0.4 0.4 0.2 0.4 0.2 Urticaria‡ Any 1.2 0.8 1.5 1.4 1.1 1.9 3.4 2.6 Mild 0.8 0.5 1.4 0.7 1.1 1.5 1.7 1.2 Moderate 0.2 0.2 0.2 0.7 0.0 0.2 1.5 1.2 Severe 0.2 0.2 0.0 0.0 0.0 0.2 0.2 0.2 Fever§, ¶ ≥38.0°C 18.4 16.4 20.4 21.7 20.0 20.0 13.3 14.0 ≥38.0°C to
<39.0°C17.3 15.7 18.5 18.1 17.2 17.2 12.1 13.2 ≥39.0°C to
<40.0°C1.0 0.7 1.6 3.4 2.4 2.5 0.8 0.8 ≥40.0°C 0.0 0.0 0.4 0.2 0.4 0.2 0.4 0.0 Across Studies 8-10 (excluding participants in Study 9 who received VAXNEUVANCE to complete a pneumococcal conjugate vaccine series initiated with Prevnar 13), the percentage of participants with fever that occurred within 7 days following administration of VAXNEUVANCE or Prevnar 13 is shown in Table 4.
Table 4: Percentage of Participants with Fever in Infants at 2, 4, 6 and 12 through 15 Months of Age After Vaccination (Studies 8-10)* Dose Dose 1 Dose 2 Dose 3 Dose 4 VAXNEUVANCE (%)
N=2,995Prevnar 13 (%)
N=1,458VAXNEUVANCE (%)
N=2,902Prevnar 13 (%)
N=1,394VAXNEUVANCE (%)
N=2,865Prevnar 13 (%)
N=1,344VAXNEUVANCE (%)
N=2,772Prevnar 13 (%)
N=1,287Following Doses 1-3, rectal temperature measurements were provided for 53.2% to 54.9% of participants and axillary temperature measurements were provided for 45.1% to 46.8% of participants, provided as a range across the doses.
Following Dose 4, rectal temperature measurements were provided for 47.0% of participants and axillary temperature measurements were provided for 53.0% of participants.
N=Number of participants with temperature data.- * Studies 8-10 (NCT03893448, NCT03620162 and NCT03692871) were randomized, double-blind, active comparator-controlled clinical studies. Licensed pediatric vaccines were administered concomitantly according to the study design or local recommended schedule.
- † Solicited on Day 1 through Day 7 postvaccination following each dose.
Fever† ≥38.0°C 15.2 12.6 19.2 18.3 17.1 16.4 15.2 13.0 ≥38.0°C to
<39.0°C14.4 11.7 17.1 15.8 14.6 14.7 12.7 11.4 ≥39.0°C to
<40.0°C0.7 0.9 2.0 2.2 2.3 1.6 1.9 1.4 ≥40.0°C 0.0 0.0 0.1 0.3 0.2 0.1 0.5 0.2 Unsolicited Adverse Reactions in Children Receiving a 4-Dose Series
Across Studies 8-11 (excluding participants in Study 9 who received VAXNEUVANCE to complete a pneumococcal conjugate vaccine series initiated with Prevnar 13), injection-site urticaria within 14 days following each dose of VAXNEUVANCE occurred in up to 0.6% of children. Participants in these studies may have received either US-licensed or non-US licensed concomitant vaccines according to the local recommended schedule.
Serious Adverse Events in Children Receiving a 4-Dose Series
Among children who received VAXNEUVANCE (N=3,349) or Prevnar 13 (N=1,814) across Studies 8-11 (excluding participants in Study 9 who received VAXNEUVANCE to complete a pneumococcal conjugate vaccine series initiated with Prevnar 13), serious adverse events up to 6 months following vaccination with the 4-dose series were reported by 9.6% of VAXNEUVANCE recipients and by 8.9% of Prevnar 13 recipients. Participants in these studies may have received either US-licensed or non-US licensed concomitant vaccines according to the local recommended schedule.
Up to 30 days following completion of Doses 1 through 3, serious adverse events were reported by 4.8% of VAXNEUVANCE recipients and by 5.0% of Prevnar 13 recipients. An adverse reaction of febrile seizure was reported in a 9 week old female (Study 11) one day after receiving VAXNEUVANCE (Dose 1) and recommended infant vaccines. Up to 30 days following Dose 4, serious adverse events were reported by 1.0% of VAXNEUVANCE recipients and by 0.7% of Prevnar 13 recipients.
There were no notable patterns or numerical imbalances between vaccination groups for specific categories of serious adverse events that would suggest a causal relationship to VAXNEUVANCE.
Safety of VAXNEUVANCE When Used to Complete a 4-Dose Pneumococcal Conjugate Vaccine Series Initiated with Prevnar 13
The safety profile observed when VAXNEUVANCE was used to complete a 4-dose pneumococcal conjugate vaccine series initiated with Prevnar 13 was similar to the safety profile following a complete 4-dose regimen of either VAXNEUVANCE or Prevnar 13 [see Clinical Studies (14.1)].
Safety Assessment in Infants and Children Receiving Catch-Up Vaccination
The safety of VAXNEUVANCE in healthy infants and children 7 months through 17 years of age was assessed in a double-blind, multi-regional, clinical study (Study 12, NCT03885934). Participants were randomized to receive 1 to 3 doses of VAXNEUVANCE (N=303) or Prevnar 13 (N=303), depending on age at enrollment. All infants and children less than 2 years of age were pneumococcal vaccine-naïve. Among 352 children 2 through 17 years of age, 42.9% had a history of previous vaccination with a lower valency pneumococcal conjugate vaccine. Among participants 7 through 11 months of age, the median age was 8.0 months, 48.4% were female, 82.8% were Asian, 17.2% were White and none were of Hispanic or Latino ethnicity. Among participants 12 through 23 months of age, the median age was 18.0 months, 54.0% were female, 83.3% were Asian, 16.7% were White and 0.8% were of Hispanic or Latino ethnicity. Among participants 2 through 17 years of age, the median age was 4.0 years, 47.7% were female, 66.8% were White, 33.0% were Asian, and none were of Hispanic or Latino ethnicity. The safety assessment was consistent with that used in Studies 8-11, as described above with the exception that in children 3 years of age and older, oral or axillary temperature measurements were obtained. The duration of the safety follow-up period for serious adverse events following the last dose of vaccine within each age cohort was 6 months.
Solicited Adverse Reactions in Children Receiving Catch-Up Vaccination
Among participants 7 through 11 months of age who received 3 doses of VAXNEUVANCE (N=64) or Prevnar 13 (N=64), the percentage of participants reporting solicited local and systemic adverse reactions that occurred within 14 days following any dose (VAXNEUVANCE participants vs. Prevnar 13 participants) were: fever ≥38.0°C (21.9% vs. 14.1%), irritability (32.8% vs. 43.8%), injection-site erythema (28.1% vs. 34.4%), somnolence (21.9% vs. 15.6%), injection-site swelling (18.8% vs. 15.6%), injection-site pain (18.8% vs. 7.8%), injection-site induration (17.2% vs. 14.1%), decreased appetite (15.6% vs. 18.8%) and urticaria (1.6% vs. 4.7%).
Among participants 12 through 23 months of age who received 2 doses of VAXNEUVANCE (N=62) or Prevnar 13 (N=64), the percentage of participants reporting solicited local and systemic adverse reactions that occurred within 14 days following any dose (VAXNEUVANCE participants vs. Prevnar 13 participants) were: fever ≥38.0°C (11.3% vs. 9.4%), irritability (35.5% vs. 21.9%), injection-site pain (33.9% vs. 23.4%), somnolence (24.2% vs. 17.2%), decreased appetite (22.6% vs. 18.8%), injection-site erythema (21.0% vs. 21.9%), injection-site swelling (14.5% vs. 12.5%) and injection-site induration (8.1% vs. 9.4%).
In children 2 through 17 years of age, the percentage of participants with solicited adverse reactions that occurred within 14 days following administration of a single dose of VAXNEUVANCE or Prevnar 13 is shown in Table 5.
Table 5: Percentage of Participants with Solicited Local and Systemic Adverse Reactions in Children and Adolescents 2 Years Through 17 Years of Age Using a Catch Up Vaccination Schedule (Study 12)* VAXNEUVANCE (%)
N=177Prevnar 13 (%)
N=175The percentage of participants 2 to <3 years of age with rectal temperature measurements was 5.0% and with axillary temperature measurements was 95.0%.
The percentage of participants ≥3 to 17 years of age with oral temperature measurements was 65.4% and with axillary temperature measurements was 34.6%.
N=Number of participants vaccinated.- * Study 12 (NCT03885934) was a randomized, double-blind, active comparator-controlled clinical study. Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination following each dose. The table represents the final assessment by the study investigators upon review of the VRC 15 days postvaccination, to ensure consistency with protocol definitions.
- † For all participants, reactions were solicited on Day 1 through Day 14 postvaccination following each dose.
- ‡ Different systemic adverse events were solicited for participants 2 to <3 years of age than for participants ≥3 to 17 years of age. For participants <3 years of age (VAXNEUVANCE N=32, Prevnar 13 N=28), decreased appetite, irritability, somnolence, and urticaria were solicited from Day 1 through Day 14 following vaccination. For participants ≥3 to 17 years of age, fatigue, headache, myalgia, arthralgia and urticaria were solicited from Day 1 through Day 14 following vaccination; no events of arthralgia were reported in VAXNEUVANCE recipients.
- § Moderate: definitely acting like something is wrong; severe: extremely distressed or unable to do usual activities.
- ¶ Solicited on Day 1 through Day 7 postvaccination following each dose.
- # Percentages reflect the number of participants with temperature data.
Local Reactions† Pain‡ Any 54.8 56.6 Moderate 27.7 22.9 Severe 4.5 1.7 Swelling Any 20.9 24.0 2.6-7.6 cm 10.2 12.0 >7.6 cm 0.0 0.6 Erythema Any 19.2 21.1 2.6-7.6 cm 6.2 7.4 >7.6 cm 1.1 0.6 Induration Any 6.8 14.9 2.6-7.6 cm 3.4 5.7 >7.6 cm 0.0 0.0 Systemic Reactions†, ‡ Myalgia§ Any 23.7 16.6 Moderate 14.7 6.9 Severe 0.6 0.6 Fatigue§ Any 15.8 17.1 Moderate 6.2 5.7 Severe 2.8 0.6 Headache§ Any 11.9 13.7 Moderate 6.2 8.6 Severe 0.6 0.6 Somnolence§ Any 2.8 2.9 Moderate 1.7 1.1 Severe 0.0 0.6 Irritability§ Any 2.8 4.0 Moderate 0.6 0.6 Severe 0.0 0.0 Decreased appetite§ Any 2.3 2.9 Moderate 0.6 1.7 Severe 0.0 0.0 Urticaria§ Any 1.1 1.1 Moderate 0.0 0.0 Severe 0.0 0.0 Fever¶, # ≥38.0°C 4.0 1.7 ≥38.0°C to <39.0°C 2.8 1.7 ≥39.0°C to <40.0°C 1.1 0.0 ≥40.0°C 0.0 0.0 Clinical Trials Experience in Adults
Safety Assessment in Clinical Studies
The safety of VAXNEUVANCE was assessed in 7 randomized, double-blind clinical studies conducted in the Americas, Europe and Asia Pacific, in which 5,630 adults 18 years of age and older received VAXNEUVANCE and 1,808 adults received Prevnar 13. In Studies 1-3 (NCT03950622, NCT03950856, and NCT03480763), a total of 3,032 adults 50 years of age and older with no history of pneumococcal vaccination received VAXNEUVANCE and 1,154 participants received Prevnar 13. In Study 4 (NCT03547167), adults 18 through 49 years of age with no history of pneumococcal vaccination, including individuals with increased risk of developing pneumococcal disease, received VAXNEUVANCE (N=1,134) or Prevnar 13 (N=378), followed by PNEUMOVAX 23 six months later. In Study 5 (NCT02573181), adults 65 years of age and older previously vaccinated with PNEUMOVAX 23 (at least 1 year prior to study entry) received VAXNEUVANCE (N=127) or Prevnar 13 (N=126). In Study 6 (NCT03615482), adults 50 years of age and older received VAXNEUVANCE concomitantly with a seasonal inactivated quadrivalent influenza vaccine (Fluarix Quadrivalent; QIV) (Group 1, N=600) or nonconcomitantly 30 days after QIV (Group 2, N=585). In this study population, 20.9% of individuals had a history of prior vaccination with PNEUMOVAX 23. In Study 7 (NCT03480802), HIV-infected adults 18 years of age and older received VAXNEUVANCE (N=152) or Prevnar 13 (N=150), followed by PNEUMOVAX 23 two months later.
The clinical studies included adults with stable underlying medical conditions (e.g., diabetes mellitus, renal disorders, chronic heart disease, chronic liver disease, chronic lung disease including asthma) and/or behavioral risk factors (e.g., smoking, increased alcohol use) that are known to increase the risk of pneumococcal disease. Overall, the mean age of the participants was 58 years and 54.6% were female. The racial distribution was as follows: 72.3% were White, 9.9% were Asian, 8.1% were American Indian or Alaska Native, 7.4% were Black or African American, and 18.1% were of Hispanic or Latino ethnicity.
In all studies, safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination. Study investigators reviewed the VRC with the participants 15 days postvaccination to ensure consistency with protocol definitions. The analyses presented in Tables 1-3 below reflect the information based on the final assessment by the study investigators. Oral body temperature and injection-site adverse reactions were solicited on Day 1 through Day 5 postvaccination. Systemic adverse reactions were solicited on Day 1 through Day 14 postvaccination. Unsolicited adverse events were reported on Day 1 through Day 14 postvaccination.
The duration of the safety follow-up period for serious adverse events postvaccination with VAXNEUVANCE was 1 month in Study 5; 2 months in Study 7; 6 months in Studies 1, 2, 4 and 6; and 12 months in Study 3.
Solicited Adverse Reactions
The percentage of participants with solicited adverse reactions that occurred within 5 or 14 days following administration of VAXNEUVANCE or Prevnar 13 in 3 studies are shown in Tables 6-8. The majority of solicited adverse reactions lasted ≤3 days.
Table 6: Percentage of Participants with Solicited Local and Systemic Adverse Reactions in Pneumococcal Vaccine-Naïve Adults 50 Years of Age and Older (Study 2)* VAXNEUVANCE (%)
N=2,103Prevnar 13 (%)
N=230N=Number of participants vaccinated. - * Study 2 (NCT03950856) was a randomized (9:1), double-blind, active comparator-controlled, lot to lot consistency study. Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination. The table represents the final assessment by the study investigators upon review of the VRC 15 days postvaccination, to ensure consistency with protocol definitions.
- † Solicited on Day 1 through Day 5 postvaccination.
- ‡ Any use of narcotic pain reliever or prevents daily activity.
- § Solicited on Day 1 through Day 14 postvaccination.
- ¶ Percentages are based on the number of participants with temperature data.
Local Reactions† Pain Any 66.8 52.2 Grade 3‡ 0.9 0.0 Erythema Any 10.9 9.6 >10 cm 0.6 0.4 Swelling Any 15.4 14.3 >10 cm 0.2 0.0 Systemic Reactions§ Fatigue Any 21.5 22.2 Grade 3‡ 0.7 0.9 Headache Any 18.9 18.7 Grade 3‡ 0.8 0.0 Myalgia Any 26.9 21.7 Grade 3‡ 0.4 0.0 Arthralgia Any 7.7 5.7 Grade 3‡ 0.2 0.0 Fever†, ¶ ≥38.0°C and <38.5°C 0.6 0.4 ≥38.5°C and <39.0°C 0.1 0.0 ≥39.0°C 0.0 0.0 Table 7: Percentage of Participants with Solicited Local and Systemic Adverse Reactions in Pneumococcal Vaccine-Naïve Adults 18 to 49 Years of Age With or Without Risk Factors for Pneumococcal Disease (Study 4)* VAXNEUVANCE (%)
N=1,134Prevnar 13 (%)
N=378N=Number of participants vaccinated. - * Study 4 (NCT03547167) was a randomized (3:1), double-blind, descriptive study. Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination. The table represents the final assessment by the study investigators upon review of the VRC 15 days postvaccination, to ensure consistency with protocol definitions.
- † Solicited on Day 1 through Day 5 postvaccination.
- ‡ Any use of narcotic pain reliever or prevents daily activity.
- § Solicited on Day 1 through Day 14 postvaccination.
- ¶ Percentages are based on the number of participants with temperature data.
Local Reactions† Pain Any 75.8 68.8 Grade 3‡ 1.1 1.6 Erythema Any 15.1 14.0 >10 cm 0.5 0.3 Swelling Any 21.7 22.2 >10 cm 0.4 0.5 Systemic Reactions§ Fatigue Any 34.3 36.8 Grade 3‡ 1.0 0.8 Headache Any 26.5 24.9 Grade 3‡ 0.8 0.5 Myalgia Any 28.8 26.5 Grade 3‡ 0.3 0.5 Arthralgia Any 12.7 11.6 Grade 3‡ 0.4 0.0 Fever†, ¶ ≥38.0°C and <38.5°C 1.0 0.3 ≥38.5°C and <39.0°C 0.3 0.0 ≥39.0°C 0.2 0.0 Table 8: Percentage of Participants with Solicited Local and Systemic Adverse Reactions in Adults 65 Years of Age and Older with Previous Pneumococcal Vaccination (Study 5)* VAXNEUVANCE (%)
N=127Prevnar 13 (%)
N=126N=Number of participants vaccinated. - * Study 5 (NCT02573181) was a randomized, double-blind, descriptive study. Safety was monitored using a Vaccination Report Card (VRC) for up to 14 days postvaccination. The table represents the final assessment by the study investigators upon review of the VRC 15 days postvaccination, to ensure consistency with protocol definitions.
- † Solicited on Day 1 through Day 5 postvaccination.
- ‡ Any use of narcotic pain reliever or prevents daily activity.
- § Solicited on Day 1 through Day 14 postvaccination.
- ¶ Percentages are based on the number of participants with temperature data.
Local Reactions† Pain Any 55.1 44.4 Grade 3‡ 0.8 0.0 Erythema Any 7.9 7.1 >10 cm 0.8 0.0 Swelling Any 14.2 6.3 >10 cm 0.0 0.0 Systemic Reactions§ Fatigue Any 18.1 19.0 Grade 3‡ 0.0 0.0 Headache Any 13.4 15.9 Grade 3‡ 0.0 0.0 Myalgia Any 15.7 11.1 Grade 3‡ 0.8 0.0 Arthralgia Any 5.5 8.7 Grade 3‡ 0.0 0.0 Fever†, ¶ ≥38.0°C and <38.5°C 1.6 0.0 ≥38.5°C and <39.0°C 0.0 0.0 ≥39.0°C 0.0 0.0 Unsolicited Adverse Reactions
Across all studies, injection-site pruritus was reported to occur in up to 2.8% of adults vaccinated with VAXNEUVANCE.
Serious Adverse Events
Across all studies, among participants 18 years of age and older who received VAXNEUVANCE (excluding those who received QIV concomitantly; N=5,030) or Prevnar 13 (N=1,808), serious adverse events within 30 days postvaccination were reported by 0.4% of VAXNEUVANCE recipients and by 0.7% of Prevnar 13 recipients. In a subset of these studies, among those who received VAXNEUVANCE (N=4,751) and Prevnar 13 (N=1,532), serious adverse events within 6 months postvaccination were reported by 2.5% of VAXNEUVANCE recipients and by 2.4% of Prevnar 13 recipients.
There were no notable patterns or numerical imbalances between vaccination groups for specific categories of serious adverse events that would suggest a causal relationship to VAXNEUVANCE.
Safety with Concomitant Influenza Vaccine Administration
The safety profile was similar when VAXNEUVANCE was administered with or without inactivated quadrivalent influenza vaccine.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
There are no adequate and well-controlled studies of VAXNEUVANCE in pregnant women. Available data on VAXNEUVANCE administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
Developmental toxicity studies have been performed in female rats administered a human dose of VAXNEUVANCE on four occasions; twice prior to mating, once during gestation and once during lactation. These studies revealed no evidence of harm to the fetus due to VAXNEUVANCE [see Animal Data below].
Data
Developmental toxicity studies have been performed in female rats. In these studies, female rats received a human dose of VAXNEUVANCE by intramuscular injection on day 28 and day 7 prior to mating, and on gestation day 6 and on lactation day 7. No vaccine related fetal malformations or variations were observed. No adverse effect on pup weight up to post-natal day 21 was noted.
8.2 Lactation
Risk Summary
Human data are not available to assess the impact of VAXNEUVANCE on milk production, its presence in breast milk, or its effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VAXNEUVANCE and any potential adverse effects on the breastfed child from VAXNEUVANCE or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of VAXNEUVANCE have been established in individuals 6 weeks through 17 years of age [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. The safety and effectiveness of VAXNEUVANCE in individuals younger than 6 weeks of age have not been established.
8.5 Geriatric Use
Of the 4,389 individuals aged 50 years and older who received VAXNEUVANCE, 2,478 (56.5%) were 65 years and older, and 479 (10.9%) were 75 years and older [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. Overall, there were no clinically meaningful differences in the safety profile or immune responses observed in older individuals (65 to 74 years and 75 years of age and older) when compared to younger individuals.
8.6 Individuals at Increased Risk for Pneumococcal Disease
Infants Born Prematurely
The safety and immunogenicity of VAXNEUVANCE were evaluated in preterm infants (<37 weeks gestation at birth) who were randomized to receive a complete 4-dose series of either VAXNEUVANCE (N=142) or Prevnar 13 (N=144) within Study 8, Study 9, and Study 10. Participants in these studies may have received either US-licensed or non-US licensed concomitant vaccines according to the local recommended schedule. In descriptive analyses, serotype-specific immunoglobulin G (IgG) and opsonophagocytic activity (OPA) responses at 30 days postdose 3, predose 4 and at 30 days postdose 4 were numerically similar between vaccination groups for the 13 shared serotypes and higher in VAXNEUVANCE for the 2 unique serotypes. The safety profile of VAXNEUVANCE was similar to the safety profile of Prevnar 13. In addition, the immune responses and safety profile in preterm infants receiving a 4-dose series of VAXNEUVANCE were similar to those observed in term infants in these studies. The effectiveness of VAXNEUVANCE in infants born prematurely has not been established.
Children with Sickle Cell Disease
In a double-blind, descriptive study (Study 13, NCT03731182), the safety and immunogenicity of VAXNEUVANCE were evaluated in children 5 through 17 years of age with sickle cell disease. Participants were randomized 2:1 to receive a single dose of VAXNEUVANCE (N=70) or Prevnar 13 (N=34). Immune responses were assessed by serotype-specific IgG GMCs and OPA GMTs at 30 days postvaccination for all 15 serotypes contained in VAXNEUVANCE. For all vaccine serotypes included in VAXNEUVANCE, serotype-specific IgG GMCs and OPA GMTs were higher following vaccination compared to pre-vaccination. IgG GMCs and OPA GMTs were numerically similar between the two vaccination groups for the 13 shared serotypes and higher in VAXNEUVANCE for serotypes 22F and 33F. The safety profile of VAXNEUVANCE was similar to the safety profile of Prevnar 13. The effectiveness of VAXNEUVANCE in children with sickle cell disease has not been established.
Individuals with HIV Infection
Children with HIV Infection
In a double-blind, descriptive study (Study 14, NCT03921424), the safety and immunogenicity of VAXNEUVANCE were evaluated in HIV-infected children 6 through 17 years of age, with CD4+ T-cell count ≥200 cells per microliter and plasma HIV RNA value <50,000 copies/mL. Participants were randomized to receive a single dose of VAXNEUVANCE (N=203) or Prevnar 13 (N=204), followed by PNEUMOVAX 23 two months later. For all vaccine serotypes included in VAXNEUVANCE, serotype-specific IgG GMCs and OPA GMTs were higher following vaccination compared to pre-vaccination. Serotype-specific IgG GMCs and OPA GMTs were numerically similar for the 13 shared serotypes and higher for the 2 unique serotypes (22F and 33F) at 30 days following vaccination with VAXNEUVANCE or Prevnar 13 and were numerically similar for all 15 serotypes contained in VAXNEUVANCE at 30 days following subsequent vaccination with PNEUMOVAX 23. The safety profile of VAXNEUVANCE was similar to the safety profile of Prevnar 13. The effectiveness of VAXNEUVANCE in HIV-infected children has not been established.
Adults with HIV Infection
In a double-blind, descriptive study (Study 7), the safety and immunogenicity of VAXNEUVANCE were evaluated in pneumococcal vaccine-naïve HIV-infected adults 18 years of age and older, with CD4+ T-cell count ≥50 cells per microliter and plasma HIV RNA value <50,000 copies/mL. Participants were randomized to receive VAXNEUVANCE (N=152) or Prevnar 13 (N=150), followed by PNEUMOVAX 23 two months later [see Adverse Reactions (6.1)]. Anti-pneumococcal opsonophagocytic activity (OPA) geometric mean antibody titers (GMTs) were higher after administration of VAXNEUVANCE, compared to pre-vaccination, for the 15 serotypes contained in VAXNEUVANCE. After sequential administration with PNEUMOVAX 23, OPA GMTs observed at 30 days after PNEUMOVAX 23 vaccination were numerically similar between the two vaccination groups for all 15 serotypes contained in VAXNEUVANCE. The safety profile of VAXNEUVANCE was similar to the safety profile of Prevnar 13. The effectiveness of VAXNEUVANCE in HIV-infected adults has not been established.
Individuals with Hematopoietic Stem Cell Transplant
In a double-blind, descriptive study (Study 15, NCT03565900), the safety and immunogenicity of VAXNEUVANCE compared to Prevnar 13 were evaluated in participants who had received an allogeneic hematopoietic stem cell transplant (allo-HSCT) 3 to 6 months prior to enrollment. All participants had a history of stable engraftment and none had severe graft-versus-host disease. In this study, participants were randomized to receive 3 doses of VAXNEUVANCE (N=139) or Prevnar 13 (N=138), administered one month apart. Among those participants 3 through 17 years of age, 8 participants received VAXNEUVANCE and 6 participants received Prevnar 13. The remaining participants were 18 through 74 years of age. Twelve months after allo-HSCT, participants without chronic graft-versus-host disease (cGVHD) received a single dose of PNEUMOVAX 23 (N=164) and those with cGVHD received a fourth consecutive dose of VAXNEUVANCE (N=29) or Prevnar 13 (N=37). IgG GMCs and OPA GMTs were higher after administration of 3 doses of VAXNEUVANCE, compared to pre-vaccination, for the 15 serotypes contained in VAXNEUVANCE. Serotype-specific IgG GMCs and OPA GMTs were numerically similar between the two vaccination groups for the 13 shared serotypes and higher in VAXNEUVANCE for the two unique serotypes (22F and 33F). Similarly, in participants who received VAXNEUVANCE or Prevnar 13 twelve months after allo-HSCT, IgG GMCs and OPA GMTs at 30 days following vaccination were numerically similar between the two vaccination groups for the 13 shared serotypes and higher in VAXNEUVANCE for the two unique serotypes (22F and 33F). In participants who received PNEUMOVAX 23 twelve months after allo-HSCT, IgG GMCs and OPA GMTs at 30 days following vaccination were numerically similar between those who had received either 3 doses of VAXNEUVANCE or Prevnar 13 for all 15 serotypes contained in VAXNEUVANCE. The safety profile of VAXNEUVANCE was similar to the safety profile of Prevnar 13. The effectiveness of VAXNEUVANCE in recipients of allo-HSCT has not been established.
-
11 DESCRIPTION
VAXNEUVANCE (Pneumococcal 15-valent Conjugate Vaccine) is a sterile suspension of purified capsular polysaccharides from S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F individually conjugated to CRM197. Each pneumococcal capsular polysaccharide is activated via sodium metaperiodate oxidation and then individually conjugated to CRM197 carrier protein via reductive amination. CRM197 is a non-toxic variant of diphtheria toxin (originating from Corynebacterium diphtheriae C7) expressed recombinantly in Pseudomonas fluorescens.
Each of the fifteen serotypes is manufactured independently using the same manufacturing steps with slight variations to accommodate for differences in strains, polysaccharides and process stream properties. Each S. pneumoniae serotype is grown in media containing yeast extract, dextrose, salts and soy peptone. Each polysaccharide is purified by a series of chemical and physical methods. Then each polysaccharide is chemically activated and conjugated to the carrier protein CRM197 to form each glycoconjugate. CRM197 is isolated from cultures grown in a glycerol-based, chemically-defined, salt medium and purified by chromatography and ultrafiltration. The final vaccine is prepared by blending the fifteen glycoconjugates with aluminum phosphate adjuvant in a final buffer containing histidine, polysorbate 20 and sodium chloride.
Each 0.5 mL dose contains 2.0 mcg each of S. pneumoniae polysaccharide serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F, and 4.0 mcg of polysaccharide serotype 6B, 30 mcg of CRM197 carrier protein, 1.55 mg L-histidine, 1 mg of polysorbate 20, 4.50 mg sodium chloride, and 125 mcg of aluminum as aluminum phosphate adjuvant. VAXNEUVANCE does not contain any preservatives.
The tip cap and plunger stopper of the prefilled syringe are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Protection against invasive disease is conferred mainly by antibodies (Immunoglobulin G [IgG] directed against capsular polysaccharides) and opsonophagocytic activity (OPA) against S. pneumoniae. VAXNEUVANCE induces IgG antibodies and OPA against the serotypes contained in the vaccine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
VAXNEUVANCE has not been evaluated for carcinogenic or mutagenic potential or for impairment of male fertility in animals. VAXNEUVANCE administered to female rats had no effect on fertility [see Use in Specific Populations (8.1)].
-
14 CLINICAL STUDIES
Immune responses elicited by VAXNEUVANCE and Prevnar 13 in children were measured by a pneumococcal electrochemiluminescence (Pn ECL) assay for total IgG and a multiplexed opsonophagocytic assay (MOPA) for opsonophagocytic killing for the 15 pneumococcal serotypes contained in VAXNEUVANCE postdose 3, predose 4 and postdose 4. In children, a serotype-specific Immunoglobulin G (IgG) antibody level corresponding to ≥0.35 mcg/mL using the WHO enzyme linked immunosorbent assay (ELISA) has been used as the threshold value for the clinical evaluation of pneumococcal conjugate vaccines. Immune responses elicited by VAXNEUVANCE and Prevnar 13 in adults were measured by MOPA and Pn ECL assays for the 15 pneumococcal serotypes contained in VAXNEUVANCE pre- and post-vaccination.
14.1 Clinical Trials in Children
Children Receiving a 4-Dose Series
In a double-blind, active comparator-controlled study (Study 8), participants were randomized to receive VAXNEUVANCE (N=860) or Prevnar 13 (N=860) in a 4-dose series; the first 3 doses were administered to infants at 2, 4, and 6 months of age and the fourth dose was administered to children 12 through 15 months of age. Pentacel (US participants) or a non-US-licensed DTaP-IPV-Hib vaccine (non-US participants), RECOMBIVAX HB, and RotaTeq were administered concomitantly with each of the 3 infant doses. VAQTA, M-M-R II, VARIVAX, and Hiberix were administered concomitantly with the fourth dose. [See Adverse Reactions (6.1) and Clinical Studies (14.3).]
Study 8 assessed serotype-specific IgG response rates, IgG geometric mean concentrations (GMCs), and opsonophagocytic activity (OPA) geometric mean titers (GMTs), for all 15 serotypes contained in VAXNEUVANCE. At 30 days postdose 3, VAXNEUVANCE was noninferior to Prevnar 13 for the 13 shared serotypes, as assessed by the proportion of participants meeting the serotype-specific IgG threshold value of ≥0.35 mcg/mL (response rate). VAXNEUVANCE was noninferior for the 2 unique vaccine serotypes, as assessed by the IgG response rates for serotypes 22F and 33F compared with the response rate for serotype 6B (the lowest response rate for any of the shared serotypes in Prevnar 13 among US participants, excluding serotype 3) at 30 days postdose 3 (Table 9).
Table 9: Proportions of US Participants with IgG Response Rates ≥0.35 mcg/mL at 30 Days Following Dose 3 in Infants Administered VAXNEUVANCE at 2, 4 and 6 Months of Age (Study 8) Pneumococcal
SerotypeVAXNEUVANCE
(n=452-455)Prevnar 13
(n=426-430)Percentage Point Difference
(VAXNEUVANCE – Prevnar 13)
(95% CI)*, †Observed Response
PercentageObserved Response
Percentagen=Number of participants contributing to the analysis.
CI=Confidence interval; IgG=Immunoglobulin G.- * CIs are based on the Miettinen & Nurminen method.
- † A conclusion of non-inferiority of VAXNEUVANCE to Prevnar 13 is based on the lower bound of the 2-sided 95% CI for the difference in percentages (VAXNEUVANCE - Prevnar 13) being >-10 percentage points.
- ‡ A conclusion of non-inferiority of VAXNEUVANCE to Prevnar 13 is based on the comparison of the response rate for the 2 additional serotypes to the lowest responding Prevnar 13 serotype (serotype 6B), excluding serotype 3.
Serotype 1 93.8 98.6 -4.8 (-7.5, -2.4) 3 93.1 74.0 19.1 (14.4, 24.0) 4 94.7 98.1 -3.4 (-6.1, -1.0) 5 93.4 96.0 -2.6 (-5.7, 0.3) 6A 92.7 99.3 -6.6 (-9.4, -4.2) 6B 86.7 89.9 -3.2 (-7.5, 1.1) 7F 98.7 100.0 -1.3 (-2.9, -0.4) 9V 96.7 97.2 -0.5 (-2.9, 1.9) 14 97.8 98.1 -0.3 (-2.4, 1.7) 18C 96.2 98.1 -1.9 (-4.3, 0.3) 19A 97.4 99.8 -2.4 (-4.3, -1.0) 19F 98.5 100.0 -1.5 (-3.2, -0.6) 23F 89.8 91.4 -1.5 (-5.4, 2.4) Additional Serotypes 22F 98.0 ‡ 8.1 (5.1, 11.5) 33F 84.8 ‡ -5.1 (-9.5, -0.7) At 30 days postdose 3, serotype-specific IgG GMCs in the VAXNEUVANCE group were noninferior to Prevnar 13 for 12 of the 13 shared serotypes, except for serotype 6A. The IgG response to serotype 6A missed the prespecified noninferiority criterion by a small margin (the lower bound of the 2-sided 95% CI for the GMC ratio [VAXNEUVANCE/Prevnar 13] being 0.48 versus >0.5). VAXNEUVANCE was noninferior to Prevnar 13 for the 2 unique serotypes, as assessed by serotype-specific IgG GMCs for serotypes 22F and 33F compared with the IgG GMCs for serotype 4 (the lowest IgG GMC for any of the shared serotypes in Prevnar 13 among US participants, excluding serotype 3) (Table 10).
Table 10: Serotype-Specific IgG GMCs at 30 Days Following Dose 3 in US Infants Administered VAXNEUVANCE at 2, 4 and 6 Months of Age (Study 8) Pneumococcal
SerotypeVAXNEUVANCE
(n=452-455)Prevnar 13
(n=426-430)GMC Ratio*
(VAXNEUVANCE/Prevnar 13)
(95% CI)*, †GMC GMC n=Number of participants contributing to the analysis.
CI=Confidence interval; GMC=Geometric mean concentration (mcg/mL); IgG=Immunoglobulin G.- * GMC ratio and CI are calculated using the t-distribution with the variance estimate from a serotype-specific linear model utilizing the natural log-transformed antibody concentrations as the response and a single term for vaccination group.
- † A conclusion of non-inferiority of VAXNEUVANCE to Prevnar 13 is based on the lower bound of the 2-sided 95% CI for the GMC ratio (VAXNEUVANCE/Prevnar 13) being >0.5.
- ‡ A conclusion of non-inferiority of VAXNEUVANCE to Prevnar 13 is based on the comparison of the GMC for the 2 additional serotypes to the lowest responding Prevnar 13 serotype (serotype 4), excluding serotype 3.
Serotype 1 1.02 1.54 0.66 (0.61, 0.73) 3 0.96 0.56 1.70 (1.54, 1.86) 4 1.07 1.11 0.97 (0.89, 1.06) 5 1.29 1.69 0.76 (0.68, 0.85) 6A 1.33 2.48 0.53 (0.48, 0.60) 6B 1.42 1.58 0.90 (0.76, 1.06) 7F 2.17 2.83 0.77 (0.70, 0.84) 9V 1.47 1.48 1.00 (0.90, 1.10) 14 4.17 5.57 0.75 (0.66, 0.85) 18C 1.29 1.55 0.83 (0.76, 0.91) 19A 1.39 1.88 0.74 (0.67, 0.82) 19F 1.82 2.33 0.78 (0.72, 0.85) 23F 1.09 1.23 0.89 (0.79, 1.01) Additional Serotypes 22F 4.01 ‡ 3.63 (3.26, 4.04) 33F 1.38 ‡ 1.25 (1.09, 1.44) At 30 days postdose 4, serotype-specific IgG GMCs for VAXNEUVANCE were noninferior to Prevnar 13 for all 13 shared serotypes (the lower bound of the 2-sided 95% CI for the GMC ratio [VAXNEUVANCE/Prevnar 13] being >0.5) and for the 2 unique serotypes 22F and 33F as assessed by the IgG GMCs for serotypes 22F and 33F compared with the IgG GMCs for serotype 4 (the lowest IgG GMC for any of the shared serotypes in Prevnar 13 among US participants, excluding serotype 3) (Table 11).
Table 11: Serotype-Specific IgG GMCs at 30 Days Following Dose 4 in US Infants Administered VAXNEUVANCE at 2, 4, 6 and 12 to 15 Months of Age (Study 8) Pneumococcal
SerotypeVAXNEUVANCE
(n=466-470)Prevnar 13
(n=443-447)GMC Ratio*
(VAXNEUVANCE/Prevnar 13)
(95% CI)*, †GMC GMC n=Number of participants contributing to the analysis.
CI=Confidence interval; GMC=Geometric mean concentration (mcg/mL); IgG=Immunoglobulin G.- * GMC ratios and CIs are calculated using the t-distribution with the variance estimate from a serotype-specific linear model utilizing the natural log-transformed antibody concentrations as the response and a single term for vaccination group.
- † A conclusion of non-inferiority of VAXNEUVANCE to Prevnar 13 is based on the lower bound of the 2-sided 95% CI for the GMC ratio (VAXNEUVANCE/Prevnar 13) being >0.5.
- ‡ A conclusion of non-inferiority of VAXNEUVANCE to Prevnar 13 is based on the comparison of the GMC for the 2 additional serotypes to the lowest responding Prevnar 13 serotype (serotype 4), excluding serotype 3.
Serotype 1 1.21 1.82 0.66 (0.60, 0.73) 3 0.91 0.63 1.43 (1.30, 1.57) 4 1.07 1.42 0.76 (0.68, 0.84) 5 2.21 3.47 0.64 (0.57, 0.71) 6A 3.56 5.93 0.60 (0.54, 0.67) 6B 4.70 6.07 0.77 (0.69, 0.87) 7F 3.22 4.65 0.69 (0.62, 0.77) 9V 2.18 2.86 0.76 (0.69, 0.84) 14 5.09 6.21 0.82 (0.72, 0.93) 18C 2.37 2.59 0.92 (0.82, 1.02) 19A 3.86 4.93 0.78 (0.71, 0.86) 19F 3.32 4.02 0.83 (0.75, 0.91) 23F 1.85 2.88 0.64 (0.57, 0.72) Additional Serotypes 22F 6.76 ‡ 4.77 (4.28, 5.32) 33F 3.80 ‡ 2.68 (2.40, 3.00) Additionally, IgG response rates and IgG GMCs at 30 days postdose 3 and IgG GMCs at 30 days postdose 4 were statistically significantly greater for VAXNEUVANCE compared to Prevnar 13 for serotype 3 and the 2 unique serotypes (22F, 33F).
Serotype-specific OPA GMTs and response rates at 30 days postdose 3 and OPA GMTs at 30 days postdose 4 were descriptively evaluated in a subset of participants in Study 8. Serotype-specific OPA GMTs and response rates were numerically similar across groups for the 13 shared serotypes and higher in the VAXNEUVANCE group for the 2 unique serotypes.
Children Receiving VAXNEUVANCE to Complete a 4-Dose Series Initiated with Prevnar 13
In a double-blind, active comparator-controlled, descriptive study (Study 9), participants were randomized in a 1:1:1:1:1 ratio to one of five vaccination groups. Two vaccination groups received a 4-dose series composed entirely of either VAXNEUVANCE (N=180) or Prevnar 13 (N=179). The remaining 3 study groups received either 1, 2, or 3 doses of Prevnar 13 followed by VAXNEUVANCE to complete the 4-dose series (N=180, 180, and 181, respectively). Participants also received other pediatric vaccines concomitantly [see Adverse Reactions (6.1) and Clinical Studies (14.3)]. Serotype-specific IgG GMCs for the 13 shared serotypes at 30 days postdose 4 were numerically similar for participants completing the vaccination series with VAXNEUVANCE compared to participants who received a complete series with Prevnar 13.
Children and Adolescents Receiving Catch-Up Vaccination
In a double-blind, active comparator-controlled, descriptive study (Study 12), participants were enrolled in three age cohorts (7 through 11 months of age, 12 through 23 months of age, and 2 through 17 years of age) and randomized to receive VAXNEUVANCE (N=303) or Prevnar 13 (N=303). Children in the two youngest age cohorts were pneumococcal vaccine-naïve at enrollment. Children in the oldest age cohort (2 through 17 years of age) were either pneumococcal vaccine-naïve, not fully vaccinated, or had completed a dosing regimen with a lower valency pneumococcal conjugate vaccine (excluding Prevnar 13). Participants who were pneumococcal vaccine-naïve at enrollment received 1 to 3 doses of VAXNEUVANCE or Prevnar 13, depending on age at enrollment and according to the schedule shown in Table 1. All participants 2 through 17 years of age received one dose of VAXNEUVANCE. Catch-up vaccination with VAXNEUVANCE elicited immune responses, as assessed by serotype-specific IgG GMCs at 30 days following the last dose of vaccine, in children 7 months through 17 years of age that were numerically similar to Prevnar 13 for the shared serotypes and higher than Prevnar 13 for the unique serotypes 22F and 33F. Within each age cohort, serotype-specific IgG GMCs at 30 days following the last dose of vaccine were numerically similar between the vaccination groups for the 13 shared serotypes and higher in VAXNEUVANCE for the 2 unique serotypes.
14.2 Clinical Trials in Pneumococcal Vaccine-Naïve Adults
Study 1
Study 1 assessed serotype-specific opsonophagocytic activity (OPA) responses for each of the 15 serotypes contained in VAXNEUVANCE at 30 days postvaccination in a double-blind, active comparator-controlled study that enrolled pneumococcal vaccine-naïve participants 50 years of age and older. Participants were randomized to receive either VAXNEUVANCE (N=604) or Prevnar 13 (N=601) at sites in USA, Canada, Spain, Taiwan, and Japan. The mean age of participants was 66 years and 57.3% were female. The racial distribution was as follows: 67.7% were White, 25.1% were Asian, 6.1% were Black or African American and 22.0% were of Hispanic or Latino ethnicity.
Table 12 summarizes the OPA geometric mean antibody titers (GMTs) at 30 days postvaccination for the 15 serotypes contained in VAXNEUVANCE. The study demonstrated that VAXNEUVANCE is noninferior to Prevnar 13 for the 13 shared serotypes and induces statistically significantly greater OPA GMTs compared to Prevnar 13 for shared serotype 3 and for the 2 unique serotypes (22F, 33F).
Table 12: Serotype-Specific OPA GMTs in Pneumococcal Vaccine-Naïve Adults 50 Years of Age and Older (Study 1) Pneumococcal
SerotypeVAXNEUVANCE
(N = 602)Prevnar 13
(N = 600)GMT Ratio*
(VAXNEUVANCE/Prevnar 13)
(95% CI)*n GMT* n GMT* N=Number of participants randomized and vaccinated; n=Number of participants contributing to the analysis that had at least one pre-dose OPA measurement (VAXNEUVANCE, n=537-597; Prevnar 13, n=545-595) or post-dose OPA measurement (VAXNEUVANCE, n=568-580; Prevnar 13, n=528-574).
CI=confidence interval; cLDA=constrained longitudinal data analysis; GMT=geometric mean titer; OPA=opsonophagocytic activity.- * GMTs, GMT ratio, and 95% CI are estimated from a cLDA model.
- † Non-inferiority for the 13 shared serotypes was met if the lower bound of the 95% CI for the GMT ratio (VAXNEUVANCE/Prevnar 13) was > 0.5.
- ‡ Statistically significantly greater OPA GMT for serotype 3 was based on the lower bound of the 95% CI for the estimated GMT ratio (VAXNEUVANCE/Prevnar 13) > 1.2.
- § Statistically significantly greater OPA GMTs for serotypes 22F and 33F was based on the lower bound of the 95% CI for the estimated GMT ratio (VAXNEUVANCE/Prevnar 13) > 2.0.
Serotype† 1 598 257 598 321 0.80 (0.66, 0.97) 3‡ 598 215 598 133 1.62 (1.40, 1.87) 4 598 1109 598 1633 0.68 (0.57, 0.80) 5 598 445 598 560 0.79 (0.64, 0.98) 6A 596 5371 596 5276 1.02 (0.85, 1.22) 6B 598 3984 598 3179 1.25 (1.04, 1.51) 7F 596 4575 596 5830 0.78 (0.68, 0.90) 9V 598 1809 597 2193 0.83 (0.71, 0.96) 14 598 1976 598 2619 0.75 (0.64, 0.89) 18C 598 2749 598 2552 1.08 (0.91, 1.27) 19A 598 3177 597 3921 0.81 (0.70, 0.94) 19F 598 1688 598 1884 0.90 (0.77, 1.04) 23F 598 2029 598 1723 1.18 (0.96, 1.44) Additional Serotypes§ 22F 594 2381 585 73 32.52 (25.87, 40.88) 33F 598 8010 597 1114 7.19 (6.13, 8.43) Study 3
In a double-blind, active comparator-controlled, descriptive study (Study 3), pneumococcal vaccine-naïve adults 50 years of age and older were randomized to receive either VAXNEUVANCE (N=327) or Prevnar 13 (N=325), followed by PNEUMOVAX 23 one year later.
Following vaccination with PNEUMOVAX 23, OPA GMTs were numerically similar between the two vaccination groups for the 15 serotypes in VAXNEUVANCE.
Study 4
In a double-blind, descriptive study (Study 4), adults 18 through 49 years of age, including individuals with increased risk of developing pneumococcal disease, were randomized to receive VAXNEUVANCE (N=1,135) or Prevnar 13 (N=380), followed by PNEUMOVAX 23 six months later [see Adverse Reactions (6.1)]. Among those who received VAXNEUVANCE, 620 participants had one risk factor and 228 participants had two or more risk factors for pneumococcal disease.
Table 13 presents OPA GMTs in the overall study population for each of the 15 serotypes 30 days following vaccination with VAXNEUVANCE or Prevnar 13.
Table 13: Serotype-Specific OPA GMTs in Pneumococcal Vaccine-Naïve Adults 18 through 49 Years of Age With or Without Risk Factors for Pneumococcal Disease (Study 4) Pneumococcal
SerotypeVAXNEUVANCE
(N = 1,133)Prevnar 13
(N = 379)n Observed
GMT95% CI* n Observed GMT 95% CI* N=Number of participants randomized and vaccinated; n=Number of participants contributing to the analysis.
CI=confidence interval; GMT=geometric mean titer; OPA=opsonophagocytic activity.- * The within-group 95% CIs are obtained by exponentiating the CIs of the mean of the natural log values based on the t-distribution.
Serotype 1 1004 267 (242, 295) 337 267 (220, 324) 3 990 198 (184, 214) 336 150 (129, 173) 4 1001 1401 (1294, 1517) 338 2568 (2268, 2908) 5 1003 560 (508, 618) 339 731 (613, 873) 6A 994 12763 (11772, 13838) 333 11313 (9739, 13141) 6B 999 10164 (9486, 10891) 338 6958 (5987, 8086) 7F 1004 5725 (5382, 6090) 338 7583 (6762, 8503) 9V 1000 3353 (3132, 3590) 339 3969 (3541, 4449) 14 1001 5245 (4860, 5660) 339 5863 (5191, 6623) 18C 999 5695 (5314, 6103) 339 3050 (2685, 3465) 19A 1001 5335 (4985, 5710) 339 5884 (5221, 6632) 19F 1003 3253 (3051, 3468) 339 3272 (2949, 3631) 23F 1001 4828 (4443, 5247) 337 3876 (3323, 4521) Additional Serotypes 22F 991 3939 (3654, 4246) 317 291 (221, 383) 33F 999 11734 (10917, 12612) 334 2181 (1826, 2606) Following vaccination with PNEUMOVAX 23, the OPA GMTs for the 15 serotypes in VAXNEUVANCE were numerically similar among participants who had received VAXNEUVANCE or Prevnar 13 for the first vaccination.
14.3 Concomitant Vaccination
Children
In Study 8, the concomitant administration of Pentacel with each of the 3 infant doses of either VAXNEUVANCE (N=598) or Prevnar 13 (N=601) was evaluated 30 days following the third dose; concomitant administration of single doses of VAQTA, M-M-R II, VARIVAX and Hiberix with the fourth dose of either VAXNEUVANCE or Prevnar 13 was evaluated 30 days following vaccination. There was no evidence that VAXNEUVANCE, as compared to Prevnar 13, interfered with the immune responses to these concomitantly administered vaccines. The immune responses to the antigens in Pentacel following completion of the 4-dose series were not evaluated.
In Study 9, the concomitant administration of RECOMBIVAX HB with either VAXNEUVANCE (N=124) or Prevnar 13 (N=266) was evaluated 30 days following the third dose of pneumococcal conjugate vaccine. Most infants (97.2%) received a birth dose of hepatitis B vaccine, followed by two doses of RECOMBIVAX HB administered concomitantly with VAXNEUVANCE or Prevnar 13. There was no evidence that VAXNEUVANCE, as compared to Prevnar 13, interfered with the immune response to RECOMBIVAX HB.
Adults
In a double-blind, randomized study (Study 6), adults 50 years of age and older were randomized to receive VAXNEUVANCE concomitantly administered with a seasonal inactivated quadrivalent influenza vaccine (Fluarix Quadrivalent; QIV) (Group 1, N=600) or VAXNEUVANCE 30 days after receiving QIV (Group 2, N=600) [see Adverse Reactions (6.1)]. Pneumococcal vaccine serotype OPA GMTs were evaluated 30 days after VAXNEUVANCE and influenza vaccine strain hemagglutinin inhibition assay (HAI) GMTs were evaluated 30 days after QIV. The noninferiority criteria for the comparisons of GMTs [lower limit of the 2-sided 95% confidence interval (CI) of the GMT ratio (Group 1/Group 2) >0.5] were met for the 15 pneumococcal serotypes in VAXNEUVANCE and for the 4 influenza vaccine strains tested.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VAXNEUVANCE is supplied as follows:
Carton of one 0.5 mL single-dose prefilled Luer Lock syringes with tip caps. NDC: 0006-4329-02
Carton of ten 0.5 mL single-dose prefilled Luer Lock syringes with tip caps. NDC: 0006-4329-03
-
17 PATIENT COUNSELING INFORMATION
Advise the patient, parent or guardian to read the FDA-approved patient labeling (Patient Information).
Discuss the following with the patient, parent or guardian:
- Provide the required vaccine information to the patient, parent or guardian.
- Inform the patient, parent or guardian of the benefits and risks associated with vaccination.
- Inform the patient, parent or guardian that vaccination with VAXNEUVANCE may not protect all vaccine recipients.
- Discuss the importance of completing the vaccination series unless contraindicated.
- Instruct the patient, parent or guardian to report any serious adverse reactions to their healthcare provider who in turn should report such events to the vaccine manufacturer or the U.S. Department of Health and Human Services through the Vaccine Adverse Event Reporting System (VAERS), 1-800-822-7967, or report online at www.vaers.hhs.gov .
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAU.S. license number 0002
For patent information: www.msd.com/research/patent
The trademarks depicted herein are owned by their respective companies.
Copyright © 2021-2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-v114-i-2405r004
-
PATIENT PACKAGE INSERT
Patient Information
VAXNEUVANCE ® (pronounced “VAKS-noo-vans”)
(Pneumococcal 15-valent Conjugate Vaccine)Before you get VAXNEUVANCE ®, read this information sheet and be sure you understand it. If you have questions or experience any side effects, talk to your healthcare provider. This information does not take the place of talking about VAXNEUVANCE with your healthcare provider. Your healthcare provider will decide if VAXNEUVANCE is right for you or your child.
What is VAXNEUVANCE?
- VAXNEUVANCE is a vaccine to help protect against invasive disease caused by 15 types of pneumococcus (pronounced “noo-mo-ca-cus”), a kind of bacteria. Invasive disease includes:
- an infection in the blood (bacteremia).
- an infection of the coverings of the brain and spinal cord (meningitis).
- VAXNEUVANCE is for individuals 6 weeks of age and older.
- VAXNEUVANCE will not give you or your child disease caused by pneumococcus.
- VAXNEUVANCE might not protect everyone who gets the vaccine.
Who should not get VAXNEUVANCE?
Do not get VAXNEUVANCE if you or your child:
- have or had an allergic reaction to any of the ingredients in VAXNEUVANCE or to diphtheria toxoid. (See the list of ingredients at the end of this information sheet.)
What should I tell my healthcare provider before getting VAXNEUVANCE?
Tell your healthcare provider if you or your child:
- have or had an allergic reaction to any vaccine.
- have a weak immune system (which means the body has a hard time fighting off infections).
- take medicines or treatments that might weaken the immune system (like immunosuppressants or steroids).
- are pregnant or planning to get pregnant.
- are breast-feeding.
If your child is an infant, also tell your healthcare provider if your child was born too early (prematurely).
How is VAXNEUVANCE given?
VAXNEUVANCE is given as an injection into the muscle (usually in the upper arm for adults and the upper arm or thigh for children).
Children need 4 doses of the vaccine:
- The first injection is given at 2 months old.
- The second injection is given at 4 months old.
- The third injection is given at 6 months old.
- The fourth injection is given at 12 through 15 months old.
If your child did not receive the complete series according to this schedule, your healthcare provider may suggest a catch-up schedule.
Your child may get VAXNEUVANCE at the same time they get other vaccines.
Adults need one dose of the vaccine.
What are the possible side effects of VAXNEUVANCE?
The most common side effects seen with VAXNEUVANCE in children less than 2 years of age are:
- Fever
- Pain, redness, swelling, or a lump where your child got the injection
- More fussy than usual
- More sleepy than usual
- Eating less than usual
The most common side effects seen with VAXNEUVANCE in children and adolescents 2 through 17 years of age are:
- Pain, swelling, redness or a lump where your child got the injection
- Muscle aches
- Feeling tired
- Headache
The most common side effects seen with VAXNEUVANCE in adults 18 years of age and older are:
- Pain, swelling or redness where you got the injection
- Feeling tired
- Muscle aches
- Headache
- Joint pain
In most people these side effects went away within three days.
If you or your child have any side effects that become bothersome or any other unusual symptoms that develop after getting this injection, tell your healthcare provider. Tell your healthcare provider right away if you or your child have symptoms of an allergic reaction which may include:
- Difficulty breathing
- Swelling of your face, lips, tongue or throat
- Hives
- Rash
There may be side effects that are not listed here. For more information, ask your healthcare provider.
You may also report any side effects to Merck Sharp & Dohme LLC at 1-877-888-4231 or directly to Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to www.vaers.hhs.gov .
What are the ingredients in VAXNEUVANCE?Active ingredient: Bacterial sugars from 15 types of pneumococcus each linked to a protein (CRM197). The sugars from these bacteria and the protein are not alive and do not cause disease.
Inactive ingredients: Sodium chloride, L-histidine, polysorbate 20 and aluminum (aluminum phosphate is included to help the vaccine work better).VAXNEUVANCE does not have any preservatives.
The tip cap and plunger stopper of the prefilled syringe are not made with natural rubber latex.
What if I have other questions?
If you have questions about VAXNEUVANCE, talk to your healthcare provider or call 1-800-622-4477.
- VAXNEUVANCE is a vaccine to help protect against invasive disease caused by 15 types of pneumococcus (pronounced “noo-mo-ca-cus”), a kind of bacteria. Invasive disease includes:
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2021-2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.usppi-v114-i-2405r003
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 05/2024
-
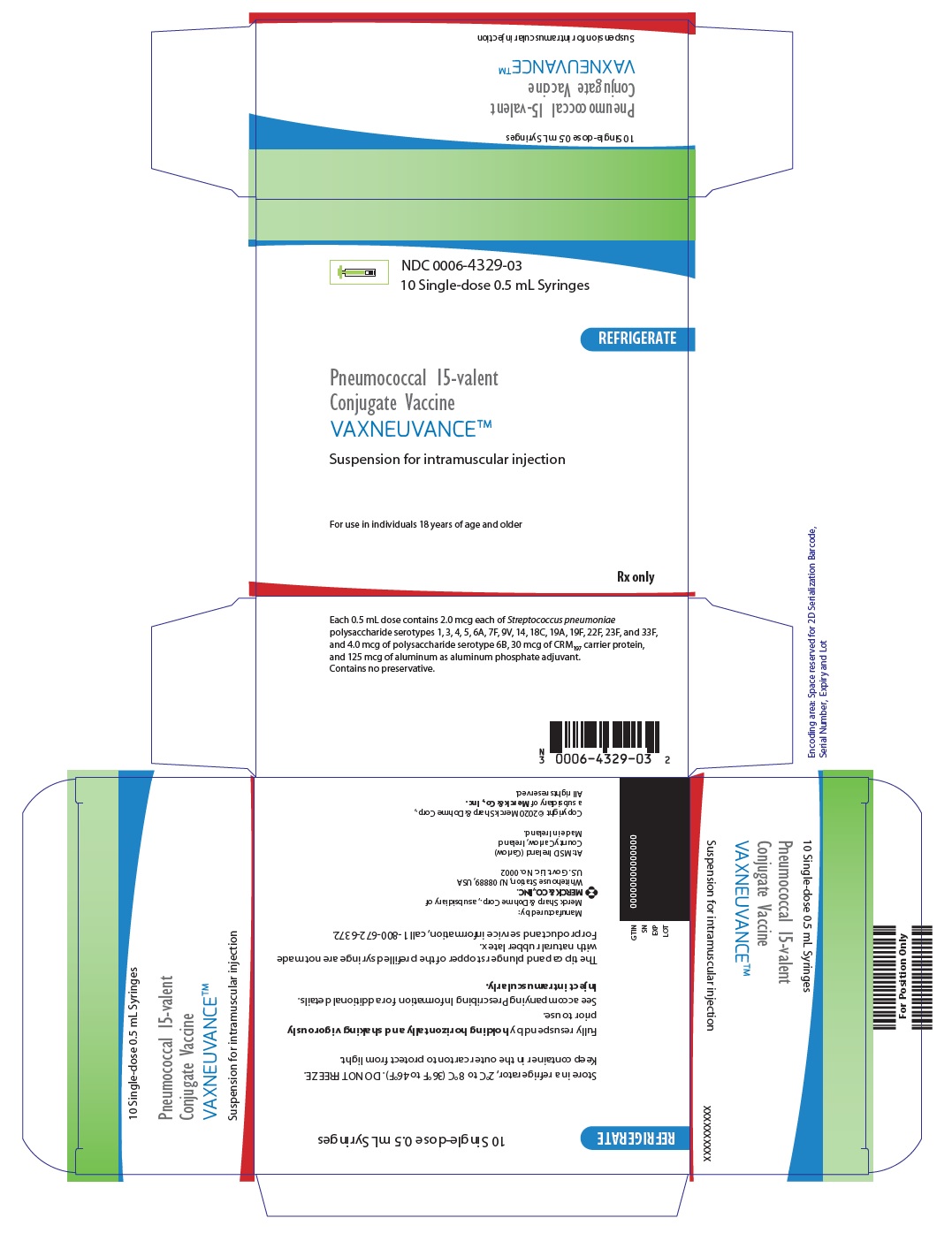
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Carton
NDC: 0006-4329-03
10 Single-dose 0.5 mL SyringesREFRIGERATE
Pneumococcal 15-valent
Conjugate Vaccine
VAXNEUVANCE™Suspension for intramuscular injection
For use in individuals 18 years of age and older
Rx only
Each 0.5 mL dose contains 2.0 mcg each of Streptococcus pneumoniae
polysaccharide serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F,
and 4.0 mcg of polysaccharide serotype 6B, 30 mcg of CRM197 carrier protein,
and 125 mcg of aluminum as aluminum phosphate adjuvant.
Contains no preservative.MERCK
-
INGREDIENTS AND APPEARANCE
VAXNEUVANCE
pneumococcal 15-valent conjugate vaccine crm197 protein adsorbed injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 0006-4329 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STREPTOCOCCUS PNEUMONIAE TYPE 1 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 54EC0SE5PZ) (STREPTOCOCCUS PNEUMONIAE TYPE 1 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:54EC0SE5PZ) STREPTOCOCCUS PNEUMONIAE TYPE 1 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 3 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 2VF3V7175U) (STREPTOCOCCUS PNEUMONIAE TYPE 3 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:2VF3V7175U) STREPTOCOCCUS PNEUMONIAE TYPE 3 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 4 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: TGJ6YZC4W7) (STREPTOCOCCUS PNEUMONIAE TYPE 4 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:TGJ6YZC4W7) STREPTOCOCCUS PNEUMONIAE TYPE 4 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 5 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 5SKG37872O) (STREPTOCOCCUS PNEUMONIAE TYPE 5 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:5SKG37872O) STREPTOCOCCUS PNEUMONIAE TYPE 5 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 6A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: Z9HK08690W) (STREPTOCOCCUS PNEUMONIAE TYPE 6A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:Z9HK08690W) STREPTOCOCCUS PNEUMONIAE TYPE 6A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 6B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 4M543JVT7G) (STREPTOCOCCUS PNEUMONIAE TYPE 6B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:4M543JVT7G) STREPTOCOCCUS PNEUMONIAE TYPE 6B CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 4 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 7F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 0K0S2P98ZJ) (STREPTOCOCCUS PNEUMONIAE TYPE 7F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:0K0S2P98ZJ) STREPTOCOCCUS PNEUMONIAE TYPE 7F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 9V CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 5Q768OY0GI) (STREPTOCOCCUS PNEUMONIAE TYPE 9V CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:5Q768OY0GI) STREPTOCOCCUS PNEUMONIAE TYPE 9V CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 14 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: SK54I0S386) (STREPTOCOCCUS PNEUMONIAE TYPE 14 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:SK54I0S386) STREPTOCOCCUS PNEUMONIAE TYPE 14 CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 18C CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: XK87D9J012) (STREPTOCOCCUS PNEUMONIAE TYPE 18C CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:XK87D9J012) STREPTOCOCCUS PNEUMONIAE TYPE 18C CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 19A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: B970MQT365) (STREPTOCOCCUS PNEUMONIAE TYPE 19A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:B970MQT365) STREPTOCOCCUS PNEUMONIAE TYPE 19A CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 19F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 2E1M7F958B) (STREPTOCOCCUS PNEUMONIAE TYPE 19F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:2E1M7F958B) STREPTOCOCCUS PNEUMONIAE TYPE 19F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 22F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: U1E9VSB2K2) (STREPTOCOCCUS PNEUMONIAE TYPE 22F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:U1E9VSB2K2) STREPTOCOCCUS PNEUMONIAE TYPE 22F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 23F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 25N8E57V6T) (STREPTOCOCCUS PNEUMONIAE TYPE 23F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:25N8E57V6T) STREPTOCOCCUS PNEUMONIAE TYPE 23F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL STREPTOCOCCUS PNEUMONIAE TYPE 33F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN (UNII: 9WP2BC3I04) (STREPTOCOCCUS PNEUMONIAE TYPE 33F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN - UNII:9WP2BC3I04) STREPTOCOCCUS PNEUMONIAE TYPE 33F CAPSULAR POLYSACCHARIDE DIPHTHERIA CRM197 PROTEIN CONJUGATE ANTIGEN 2 ug in 0.5 mL CORYNEBACTERIUM DIPHTHERIAE CRM197 PROTEIN (UNII: 08VC9WC084) (CORYNEBACTERIUM DIPHTHERIAE CRM197 PROTEIN - UNII:08VC9WC084) CORYNEBACTERIUM DIPHTHERIAE CRM197 PROTEIN 30 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALUMINUM PHOSPHATE (UNII: F92V3S521O) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-4329-03 10 in 1 CARTON 1 NDC: 0006-4329-01 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0006-4329-02 1 in 1 CARTON 2 NDC: 0006-4329-01 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125741 07/16/2021 Labeler - Merck Sharp & Dohme LLC (118446553)
Trademark Results [VAXNEUVANCE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VAXNEUVANCE 88132087 not registered Live/Pending |
Merck Sharp & Dohme Corp. 2018-09-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.