ZILXI- minocycline aerosol, foam
ZILXI by
Drug Labeling and Warnings
ZILXI by is a Prescription medication manufactured, distributed, or labeled by Journey Medical Corporation, ASM Aerosol-Service AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZILXI safely and effectively. See full prescribing information for ZILXI.
ZILXI® (minocycline) topical foam
Initial U.S. Approval: 1971INDICATIONS AND USAGE
ZILXI is a tetracycline-class drug indicated for the treatment of inflammatory lesions of rosacea in adults. (1)
Limitations of UseThis formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ZILXI should be used only as indicated (1).
DOSAGE AND ADMINISTRATION
Apply ZILXI over all areas of the face once daily. ZILXI should be gently rubbed into the skin. (2)
DOSAGE FORMS AND STRENGTHS
Foam, 1.5%. (3)
CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines or any of the ingredients in ZILXI. (4)
WARNINGS AND PRECAUTIONS
- The propellant in ZILXI is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. (5.1)
- The use of tetracycline-class of drugs orally during the second and third trimesters of pregnancy, infancy and childhood up to the age of 8 years may cause permanent discoloration of the teeth (yellow-gray-brown) and reversible inhibition of bone growth. (5.2, 5.3, 5.4, 8.4)
- If Clostridioides difficile associated diarrhea occurs, discontinue ZILXI. (5.5)
- If liver injury is suspected, discontinue ZILXI. (5.6)
- If renal impairment exists, oral minocycline doses may need to be adjusted to avoid excessive systemic accumulations of the drug and possible liver toxicity. (5.7)
- Oral minocycline may cause central nervous system side effects including lightheadedness, dizziness, or vertigo. (5.8)
- Oral minocycline may cause intracranial hypertension in adults and adolescents. Discontinue ZILXI if symptoms occur. (5.9)
- Oral minocycline has been associated with autoimmune syndromes; discontinue ZILXI immediately if symptoms occur. (5.10)
- Photosensitivity can occur with oral tetracycline. Patients should minimize or avoid exposure to natural or artificial sunlight. (5.11)
- Oral minocycline has been associated with anaphylaxis, serious skin reactions, erythema multiforme, and DRESS syndrome. Discontinue ZILXI immediately if symptoms occur. (5.12)
ADVERSE REACTIONS
The most commonly observed adverse reaction (incidence ≥1%) is diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact VYNE Pharmaceuticals Inc. at 1-844-375-3673 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Minocycline like other tetracycline-class drugs can cause fetal harm when administered orally to a pregnant woman. (5.2, 5.3, 5.4, 8.1)
- The use of drugs of the tetracycline-class orally during tooth development may cause permanent discoloration of teeth. (5.3, 8.1, 8.2, 8.4)
- Lactation: Breastfeeding not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
5.2 Teratogenic Effects
5.3 Tooth Discoloration
5.4 Inhibition of Bone Growth
5.5 Clostridioides difficile Associated Diarrhea
5.6 Hepatotoxicity
5.7 Metabolic Effects
5.8 Central Nervous System Effects
5.9 Intracranial Hypertension
5.10 Autoimmune Syndromes
5.11 Photosensitivity
5.12 Serious Skin/Hypersensitivity Reaction
5.13 Tissue Hyperpigmentation
5.14 Development of Drug-Resistant Bacteria
5.15 Superinfection/Potential for Microbial Overgrowth
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Anticoagulants
7.2 Penicillin
7.3 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ZILXI is indicated for the treatment of inflammatory lesions of rosacea in adults [see Clinical Studies (14)].
Limitations of Use
This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ZILXI should be used only as indicated [see Warnings and Precautions (5.14)].
-
2 DOSAGE AND ADMINISTRATION
For topical use only, not for oral, ophthalmic or intravaginal use.
After shaking the can well, a small amount of topical foam (e.g. a cherry-sized amount) should be expressed from the can onto the fingertips of the hand and then applied as a thin layer over all areas of the face. Additional ZILXI foam may be used as needed to ensure the entire face is treated. The topical foam should be applied at approximately the same time each day at least 1 hour before bedtime. The patient should not bathe, shower or swim for at least 1 hour after application of the product.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
The propellant in ZILXI is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. Do not puncture and/or incinerate the containers. Do not expose containers to heat and/or store at temperatures above 120°F (49°C).
5.2 Teratogenic Effects
Minocycline, like other tetracycline-class drugs, may inhibit bone growth when administered orally during pregnancy. Based on animal data, when administered orally, tetracyclines cross the placenta, are found in fetal tissues, and can cause skeletal malformation and retardation of skeletal development on the developing fetus [see Use in Specific Populations (8.1) and Nonclinical Toxicology (13.1)].
5.3 Tooth Discoloration
The use of tetracycline class drugs orally during tooth development (second and third trimesters of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term oral use of the tetracycline but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported with oral tetracycline drugs. Use of tetracycline drugs is not recommended during tooth development.
5.4 Inhibition of Bone Growth
All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that oral tetracyclines cross the placenta, are found in fetal tissues, and can cause retardation of skeletal development on the developing fetus. Evidence of embryotoxicity has been noted in animals treated orally early in pregnancy [see Use in Specific Populations (8.1)].
5.5 Clostridioides difficile Associated Diarrhea
Clostridioides difficile associated diarrhea (CDAD) has been reported with nearly all antibacterial agents, including oral minocycline, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.6 Hepatotoxicity
Post-marketing cases of serious liver injury, including irreversible drug-induced hepatitis and fulminant hepatic failure (sometimes fatal) have been reported with oral minocycline use.
5.7 Metabolic Effects
The anti-anabolic action of the tetracyclines may cause an increase in blood urea nitrogen (BUN). In patients with significantly impaired function, higher serum levels of tetracycline-class drugs may lead to azotemia, hyperphosphatemia, and acidosis. If renal impairment exists, recommended oral or parenteral doses may lead to excessive systemic accumulations of the drug and possible liver toxicity. Under such conditions, adjust the dose downward, and if therapy is prolonged, serum level determinations of the drug may be advisable.
5.8 Central Nervous System Effects
Central nervous system side effects including light-headedness, dizziness or vertigo have been reported with oral minocycline therapy. Patients who experience these symptoms should be cautioned about driving vehicles or using hazardous machinery while on minocycline therapy. These symptoms may disappear during therapy and may disappear when the drug is discontinued.
5.9 Intracranial Hypertension
Intracranial hypertension has been associated with the use of oral tetracycline-class drugs. Clinical manifestations of intracranial hypertension include headache, blurred vision, diplopia and vision loss; papilledema can be found on fundoscopy. Women of childbearing age who are overweight or have a history of IH are at a greater risk for developing intracranial hypertension. Patients should be questioned for visual disturbances prior to initiation of treatment with tetracyclines. Concomitant use of isotretinoin and tetracycline should be avoided because isotretinoin, a systemic retinoid, is also known to cause intracranial hypertension.
Although intracranial hypertension typically resolves after discontinuation of treatment, the possibility for permanent visual loss exists. If visual disturbance occurs during treatment, prompt ophthalmologic evaluation is warranted. Because intracranial pressure can remain elevated for weeks after drug cessation, patients should be monitored until they stabilize.
5.10 Autoimmune Syndromes
Tetracyclines have been associated with the development of autoimmune syndromes. The long-term use of oral minocycline has been associated with drug-induced lupus-like syndrome, autoimmune hepatitis and vasculitis. Sporadic cases of serum sickness have presented shortly after oral minocycline use. Symptoms may be manifested by fever, rash, arthralgia, and malaise. In symptomatic patients, immediately discontinue the use of all tetracycline-class drugs, including ZILXI.
5.11 Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking oral tetracyclines; this reaction has been reported less frequently with minocycline. Although ZILXI did not induce phototoxicity or photoallergic responses in human dermal safety studies, patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using minocycline. If patients need to be outdoors while using ZILXI, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Advise patients to discontinue treatment with ZILXI at the first evidence of sunburn.
5.12 Serious Skin/Hypersensitivity Reaction
Cases of anaphylaxis, serious skin reactions (e.g. Stevens Johnson syndrome), erythema multiforme, and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome have been reported postmarketing with oral minocycline use. DRESS syndrome consists of cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, and one or more of the following visceral complications such as: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis. Fever and lymphadenopathy may be present. In some cases, death has been reported with oral minocycline use. If this syndrome is recognized, discontinue ZILXI immediately.
5.13 Tissue Hyperpigmentation
Oral tetracyclines are known to cause hyperpigmentation. Tetracycline therapy may induce hyperpigmentation in many organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (teeth, mucosa, alveolar bone), sclerae and heart valves. Skin and oral pigmentation has been reported to occur independently of time or amount of drug administration, whereas other tissue pigmentation has been reported to occur upon prolonged administration. Skin pigmentation includes diffuse pigmentation as well as pigmentation over sites of scars or injury.
5.14 Development of Drug-Resistant Bacteria
ZILXI has not been evaluated in the treatment of infections. Bacterial resistance to the tetracyclines may develop in patients using ZILXI, therefore, the susceptibility of bacteria associated with infection should be considered in selecting antimicrobial therapy. Because of the potential for drug-resistant bacteria to develop during the use of ZILXI, it should be used only as indicated.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In three (two Phase 3 and one Phase 2) multicenter, randomized, double-blind, vehicle-controlled trials, adult subjects applied ZILXI or vehicle once daily for 12 weeks. A total of 1,087 subjects were treated with ZILXI and 591 with vehicle. The majority of subjects were White (97%) and female (70%). Approximately 67% were non-Hispanic/Latino. The mean age was 50.0 years and ages ranged from 18 to 86 years.
The most common adverse reaction reported by ≥1% of subjects treated with ZILXI and more frequently than in subjects treated with vehicle was diarrhea (1% vs. 0%), respectively.
During the two Phase 3 trials, local tolerability evaluations were conducted at each study visit by assessment of erythema, telangiectasia, burning/stinging, flushing/blushing, dryness, itching, peeling and hyperpigmentation. Table 1 presents local tolerance assessments by incidence rate (%) and severity grade.
Subjects treated with ZILXI had improved local tolerability signs and symptoms at Week 12 when compared with corresponding baseline values. These occurred at a similar frequency and severity as subjects treated with the vehicle component of ZILXI.
Table 1: Facial Cutaneous Tolerability Assessment *Hyperpigmentation was most frequently assessed as characteristic of inflammatory and post-inflammatory changes associated with inflammatory lesions of rosacea.
** Of 1,008 subjects, 897 had local tolerability assessments at Week 12.ZILXI, (%)
(N=1,008**)
Symptom/Severity
Mild
Moderate
Severe
Erythema
36.2
18.3
0.7
Telangiectasia
61.0
18.8
0
Burning/Stinging
13.3
2.8
0
Flushing/Blushing
39.0
9.6
0.9
Dryness
23.9
4.0
0.1
Itching
20.0
3.3
0
Skin Peeling
16.1
1.9
0.1
Hyperpigmentation*
22.5
2.8
0
In a 40-week open-label extension safety study of ZILXI (for a total of up to 52 weeks of treatment) [NCT03276936], frequency and severity of local tolerability signs and symptoms at Week 52 were comparable to those reported at Week 12.
-
7 DRUG INTERACTIONS
7.1 Anticoagulants
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with ZILXI use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Systemic absorption of ZILXI in humans is low following once daily topical administration of ZILXI under maximal clinical use conditions [see Clinical Pharmacology (12.3)]. Because of low systemic exposure, it is not expected that maternal use of ZILXI will result in significant fetal exposure to the drug.
Tetracycline-class drugs may cause permanent discoloration of teeth and reversible inhibition of bone growth when administered orally during pregnancy [see Warnings and Precautions 5.2, 5.3, 5.4).
Animal reproduction studies were not conducted with ZILXI. In animal reproduction studies, oral administration of minocycline to pregnant rats and rabbits during organogenesis induced skeletal malformations in fetuses at systemic exposures of 2,000 and 1,300 times, respectively, the maximum recommended human dose (MRHD based on AUC comparison) of ZILXI (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Results of animal studies with oral administration indicate that tetracyclines cross the placenta, are found in fetal tissues, and can cause retardation of skeletal development of the developing fetus.
Minocycline induced skeletal malformations (bent limb bones) in fetuses when orally administered to pregnant rats and rabbits during the period of organogenesis at doses of 30 mg/kg/day and 100 mg/kg/day, respectively, (2,000 times and 1,300 times, respectively, the systemic exposure at the MRHD based on AUC comparison). Reduced mean fetal body weight was observed when minocycline was orally administered to pregnant rats during the period of organogenesis at a dose of 10 mg/kg/day (680 times the systemic exposure at the MRHD based on AUC comparison).
Minocycline was assessed for effects on peri- and post-natal development of rats in a study that involved oral administration to pregnant rats during the period of organogenesis through lactation, at doses of 5, 10, or 50 mg/kg/day. In this study, body weight gain was significantly reduced in pregnant females that received 50 mg/kg/day (1,700 times the systemic exposure at the MRHD based on AUC comparison). No effects of treatment on the duration of the gestation period or the number of live pups born per litter were observed. Gross external anomalies observed in F1 pups (offspring of animals that received minocycline) included reduced body size, improperly rotated forelimbs, and reduced size of extremities. No effects were observed on the physical development, behavior, learning ability, or reproduction of F1 pups, and there was no effect on gross appearance of F2 pups (offspring of F1 animals).
8.2 Lactation
Risk Summary
Tetracycline-class drugs, including minocycline, are present in breast milk following oral administration. It is not known whether minocycline is present in human milk after topical administration to the nursing mother. There are no data on the effects of minocycline on milk production. Because of the potential for serious adverse reactions, advise patients that breastfeeding is not recommended during treatment with ZILXI [see Warnings and Precautions (5.2)].
8.4 Pediatric Use
The safety and effectiveness of ZILXI for the treatment of inflammatory lesions of rosacea have not been evaluated in pediatric patients.
8.5 Geriatric Use
There were 278 subjects aged 65 or older in the clinical trials of ZILXI (16.6% of 1,678 subjects). No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
Minocycline hydrochloride, a semi-synthetic derivative of tetracycline, is [4S‑(4α,4aα,5aα,12aα)] 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a‑tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride. The structural formula is represented below:
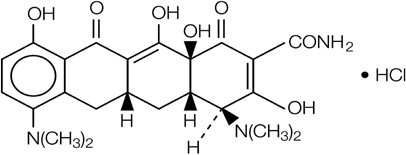
C23H27N3O7HClM. W. 493.94
Each gram of ZILXI contains micronized 15 mg minocycline equivalent to 16 mg minocycline hydrochloride in a yellow suspension foam.
In addition, the 1.5% ZILXI topical foam contains the following inactive ingredients: soybean oil, coconut oil, light mineral oil, cyclomethicone, cetostearyl alcohol, stearic acid, myristyl alcohol, hydrogenated castor oil, white wax (beeswax), stearyl alcohol, docosanol. ZILXI topical foam is dispensed from an aluminum container (can) pressurized with propellant (butane + isobutane + propane).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of ZILXI for the treatment of inflammatory lesions of rosacea is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of ZILXI for the treatment of inflammatory lesions of rosacea are unknown.
12.3 Pharmacokinetics
In a pharmacokinetic study, male and female subjects 18 years of age or older with inflammatory lesions of rosacea (N=20) applied approximately 2 grams of ZILXI topically to the face once daily for 14 days.
The mean ± SD maximum plasma concentration (Cmax) and area under the concentration time curve from 0 to 24 hours post dose (AUC0-24h) for minocycline on Day 1 were 1.3 ± 0.9 ng/mL and 22.5 ± 16.2 h·ng/mL, respectively. After daily application of ZILXI in subjects with inflammatory lesions of rosacea for 14 days, steady-state was reached by Day 1 and systemic accumulation of minocycline was not evident.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a carcinogenicity study in which minocycline hydrochloride was orally administered to male and female rats once daily for up to 104 weeks at dosages up to 200 mg/kg/day, minocycline hydrochloride was associated in both sexes with follicular cell tumors of the thyroid gland, including increased incidences of adenomas, carcinomas and the combined incidence of adenomas and carcinomas in males, and adenomas and the combined incidence of adenomas and carcinomas in females. In a carcinogenicity study in which minocycline hydrochloride was orally administered to male and female mice once daily for up to 104 weeks at dosages up to 150 mg/kg/day, exposure to minocycline hydrochloride did not result in a significantly increased incidence of neoplasms in either males or females.
Minocycline was not mutagenic in vitro in a bacterial reverse mutation assay (Ames test) or CHO/HGPRT mammalian cell assay in the presence or absence of metabolic activation. Minocycline was not clastogenic in vitro using human peripheral blood lymphocytes or in vivo in a mouse micronucleus test.
Male and female reproductive performance in rats was unaffected by oral doses of minocycline of up to 300 mg/kg/day (27,500 times the systemic exposure at the MRHD based on AUC comparison). However, oral administration of 100 or 300 mg/kg/day of minocycline to male rats (10,000 or 27,500 times, respectively, the systemic exposure at the MRHD based on AUC comparison), adversely affected spermatogenesis.
Effects observed at 300 mg/kg/day of oral minocycline included a reduced number of sperm cells per gram of epididymis, an apparent reduction in the percentage of sperm that were motile, and (at 100 and 300 mg/kg/day) increased numbers of morphologically abnormal sperm cells. Morphological abnormalities observed in sperm samples included absent heads, misshapen heads, and abnormal flagella.
-
14 CLINICAL STUDIES
The safety and efficacy of once daily use of ZILXI was assessed in two 12-week multicenter, randomized, double-blind, vehicle-controlled trials in subjects with inflammatory lesions of rosacea (Trial 1 [NCT02601963] and Trial 2 [NCT03142451]). Efficacy was assessed in a total of 1,522 subjects 18 years of age and older. ZILXI or its vehicle were applied once daily for 12 weeks; no other topical or systemic medication affecting the course of inflammatory lesions of rosacea was permitted for use during these trials.
Subjects were required to have an inflammatory lesion count in the range 15-75 lesions and an Investigator Global Assessment (IGA) score of 3 (“moderate”) or 4 (“severe”) at baseline.
Overall, 96% of subjects were White and 71% were female. Three hundred and eighty-three (25%) subjects were 18 to 40 years of age, 899 (59%) subjects were 41 to 64 years of age, and 240 (16%) subjects were 65 years or older. At baseline, subjects had a mean inflammatory lesion count of 29.4. Additionally, approximately 87% of subjects had an IGA score of 3 (“moderate”).
The co-primary efficacy endpoints were the absolute change from baseline in inflammatory lesion counts at Week 12 and the proportion of subjects with treatment success at Week 12, defined as an IGA score of 0 (“clear”) or 1 (“almost clear”), and at least a two-grade improvement (decrease) from baseline at Week 12. The efficacy results are presented in Table 2.
Table 2 Efficacy of ZILXI at Week 12 CI: Confidence Interval
(1) IGA Success is defined as an IGA score of 0 or 1 and at least a 2-grade improvement from baseline
(2) Treatment Difference and 95% CI are based on the CMH test stratified by analysis center
(3) Means presented in table are Least Square means
(4) Treatment Difference and 95% CI are based on ANCOVA model with treatment and analysis center as factors, and baseline value as covariateTrial 1
Trial 2
ZILXI
(N=495)
Vehicle
(N=256)
ZILXI
(N=514)
Vehicle
(N=257)
IGA Success (1)
52.1%
43.0%
49.1%
39.0%
Treatment Difference (95% CI) (2)
9.0%
(1.3%, 16.8%)
10.2%
(3.1%, 17.4%)
Inflammatory Lesion Count
Mean Absolute Change from Baseline (3)
-17.6
-15.4
-18.4
-14.5
Treatment Difference (95% CI) (4)
-2.2
(-3.7, -0.7)
-3.9
(-5.5, -2.2)
Mean Percent Change from Baseline (3)
-61.3%
-54.1%
-60.2%
-48.9%
Treatment Difference (95% CI) (4)
-7.3%
(-12.5%, -2.1%)
-11.3%
(-16.7%, -5.9%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ZILXI® (minocycline) topical foam, 1.5% is a yellow suspension supplied in a pressurized aluminum aerosol container (can). Each gram of ZILXI contains 15 mg of minocycline equivalent to 16 mg of minocycline hydrochloride, and is supplied as follows:
NDC: 72356-103-03 30 g Can
Storage
ZILXI must be stored at 2 ºC – 8 ºC (36 ºF – 46 ºF) until dispensed to the patient.
Once dispensed, the patient is to store ZILXI at room temperature below 25 ºC (77 ºF) for 90 days. Do not store in the refrigerator.
Handling
Allow the can to warm to room temperature before first use.
Shake can well before use.
WARNING: Flammable. Avoid fire, flame, or smoking during and immediately following application. Contents under pressure. Do not puncture or incinerate. Do not expose to heat or temperatures above 49 oC (120 oF).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Inform patients using ZILXI (minocycline) topical foam, 1.5% of the following information and instructions:
Flammability
The propellant in ZILXI is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application.
Tooth Discoloration
Advise caregivers of pediatric patients that ZILXI may cause permanent discoloration of deciduous and permanent teeth during tooth development (generally up to the age of 8 years) based on observations with oral tetracycline.
Lactation
Advise women that breastfeeding is not recommended during ZILXI therapy.
Tissue Hyperpigmentation
Inform patients that ZILXI may cause discoloration of skin, scars, teeth or gums based on observations with oral minocycline.
Clostridioides difficile Associated Diarrhea
Advise patients that Clostridioides difficile associated diarrhea can occur with oral minocycline therapy. Advise patients to seek medical attention if they develop watery or bloody stools while using ZILXI.
Hepatotoxicity
Inform patients about the possibility of hepatotoxicity reported with oral minocycline. Advise patients to seek medical advice if they experience symptoms or signs of hepatotoxicity, including loss of appetite, tiredness, diarrhea, jaundice, increased bleeding tendencies, confusion, and sleepiness.
Central Nervous System Effects
Inform patients that central nervous system adverse reactions including dizziness or vertigo have been reported with oral minocycline therapy. Caution patients about driving vehicles or using hazardous machinery if they experience such symptoms while on ZILXI.
Intracranial Hypertension
Inform patients that intracranial hypertension can occur with minocycline therapy. Advise patients to seek medical attention if they develop unusual headache, visual symptoms, such as blurred vision, diplopia, and vision loss.
Photosensitivity
Inform patients that photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking oral tetracyclines, including minocycline. Advise patients to minimize or avoid exposure to natural or artificial UV light (tanning beds or UVA/B treatment) while using ZILXI. Discuss other sun protection measures, if patients need to be outdoors while using ZILXI. Advise patients to discontinue treatment at the first evidence of sunburn.
Autoimmune Syndromes
Inform patients that autoimmune syndromes, including drug-induced lupus-like syndrome, autoimmune hepatitis, vasculitis and serum sickness have been observed with oral tetracycline-class drugs, including minocycline. Symptoms may be manifested by arthralgia, fever, rash and malaise. Advise patients who experience such symptoms to stop the drug immediately and seek medical help.
Other Information
ZILXI should be applied exactly as directed.
ZILXI may stain fabric.Manufactured by: ASM Aerosol-Service AG, Mohlin, Switzerland
Manufactured for: VYNE Pharmaceuticals Inc., Bridgewater, NJ 08807
Product of Portugal or Switzerland103G0002
ZILXI is a registered trademark of an affiliate of VYNE Therapeutics Inc.
All other trademarks are the properties of their respective owners.
Copyright © 2020, VYNE Therapeutics Inc. All rights reserved. -
PATIENT PACKAGE INSERT
PATIENT INFORMATION
ZILXI® (ZILK-see)
(minocycline)
topical foam
Important Information: ZILXI is for use on the skin only (topical use). ZILXI is not for use in your mouth, eyes, or vagina.
What is ZILXI?
ZILXI is a prescription medicine used on the skin (topical) to treat adults with pimples and bumps caused by a condition called rosacea.
ZILXI should not be used for the treatment of infections.
It is not known if ZILXI is safe and effective in children.
Do not use ZILXI if you are allergic to any tetracycline medicines or to any of the ingredients in ZILXI. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Before using ZILXI, tell your healthcare provider about all of your medical conditions, including if you:
- have diarrhea or watery stools
- have liver problems
- have kidney problems
- are pregnant or plan to become pregnant. Taking tetracycline medicines by mouth during pregnancy may cause serious side effects on the growth of bone and teeth of your baby. ZILXI topical foam is used on your skin and it is not known if it will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with ZILXI.
Tell your healthcare provider about all the other medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements, and skin products you use. Tetracycline medicines taken by mouth may affect the way other medicines work and may increase your chance of developing certain side effects.
Especially tell your healthcare provider if you take:
- a blood thinner medicine
- a penicillin antibiotic medicine
- isotretinoin
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist.
How should I use ZILXI?
- See the detailed “Instructions for Use” included with this leaflet for directions about how to apply ZILXI the right way.
- Use ZILXI exactly as your healthcare provider tells you.
- Apply ZILXI to your entire face at about the same time each day, at least 1 hour before bedtime. You should apply enough ZILXI to cover the entire face.
- Do not bathe, shower, or swim for at least 1 hour after applying ZILXI.
- Wash your hands after applying ZILXI.
What should I avoid while using ZILXI?
- ZILXI is flammable. Avoid fire, flames, and smoking when applying and right after you apply ZILXI.
- Limit your time in sunlight. Avoid sunlight or artificial sunlight such as sunlamps or tanning beds. Use sun protection measures such as sunscreen and wear loose-fitting clothes that cover your skin while out in sunlight. Stop using ZILXI if you get sunburn.
- Minocycline taken by mouth may cause feelings of light-headedness, dizziness, or spinning (vertigo). You should not drive or operate dangerous machinery if you have these symptoms during treatment with ZILXI.
What are possible side effects of ZILXI?
ZILXI contains minocycline, a tetracycline medicine. Tetracyclines, when taken by mouth, may cause serious side effects, including:
- Harm to an unborn baby. See “What should I tell my healthcare provider before using ZILXI?”
- Permanent tooth discoloration. Tetracycline medicine when taken by mouth may permanently turn a baby or child's teeth yellow-gray-brown during tooth development. You should not use ZILXI during tooth development. Tooth development happens in the second and third trimesters of pregnancy, and from birth up to 8 years of age.
- Slow bone growth. Tetracycline medicine taken by mouth may slow bone growth in infants and children. Slow bone growth is reversible after stopping treatment.
- Diarrhea. Diarrhea can happen with most antibiotics, including minocycline taken by mouth. This diarrhea may be caused by an infection (Clostridioides difficile) in your intestines. Call your healthcare provider right away if you get watery or bloody stools while using ZILXI.
- Liver problems. Minocycline taken by mouth can cause serious liver problems that may lead to death. Stop using ZILXI and call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:
o loss of appetite
o tiredness
o diarrhea
o yellowing of your skin or the white of your eyes (jaundice)
o bleeding more easily than normal
o confusion
o sleepiness
- Central nervous system effects. See “What should I avoid while using ZILXI?”
- Increased pressure in the brain (intracranial hypertension). This condition may lead to vision changes and permanent vision loss. You are more likely to get intracranial hypertension if you are a female of childbearing potential and are overweight or have a history of intracranial hypertension. Stop using ZILXI and tell your healthcare provider right away if you have blurred vision, double vision, vision loss, or unusual headaches.
- Immune system reactions including a lupus-like syndrome, hepatitis, and inflammation of blood or lymph vessels (vasculitis) have happened during treatment with minocycline taken by mouth. Call your healthcare provider right away if you get a fever, rash, joint pain, or body weakness.
- Sensitivity to sunlight (photosensitivity). See “What should I avoid while using ZILXI?”
- Serious skin or allergic reactions have happened during treatment with minocycline taken by mouth that may affect parts of your body such as your liver, lungs, kidneys and heart. Sometimes these can lead to death. Stop using ZILXI and go to the nearest hospital emergency room right away if you have any of the following signs or symptoms:o skin rash, hives, sores in your mouth, or your skin blisters and peels
o swelling of your face, eyes, lips, tongue, or throat
o trouble swallowing or breathing
o blood in your urine
o fever, yellowing of the skin or the whites of your eyes (jaundice), dark colored urine
o pain on the right side of the stomach area (abdominal pain)
o chest pain or abnormal heartbeats
o swelling in your legs, ankles, and feet
- Discoloration (hyperpigmentation). Minocycline taken by mouth may cause darkening of your skin, scars, teeth, or gums.
The most common side effect of ZILXI is diarrhea.
Your healthcare provider may stop your treatment with ZILXI if you develop certain side effects.
These are not all the possible side effects with ZILXI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZILXI?
- Store ZILXI at room temperature below 77ºF (25ºC) for 90 days.
- Do not store ZILXI in the refrigerator.
- Do not puncture or burn the ZILXI can.
- Do not expose to heat or temperatures above 120ºF (49ºC)
Keep ZILXI and all medicines out of the reach of children.
General information about the safe and effective use of ZILXI.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflet. Do not use ZILXI for a condition for which it was not prescribed. Do not give ZILXI to other people, even if they have the same symptoms you have. It may harm them. You can also ask your healthcare provider or pharmacist for information about ZILXI that is written for health professionals.
What are the ingredients in ZILXI?
Active ingredient: minocycline
Inactive ingredients: soybean oil, coconut oil, light mineral oil, cyclomethicone, cetostearyl alcohol, stearic acid, myristyl alcohol, hydrogenated castor oil, white wax (beeswax), stearyl alcohol, docosanol. ZILXI is dispensed from an aluminum container (can) pressurized with propellant (butane + isobutane + propane).
Manufactured by: ASM Aerosol-Service AG, Mohlin, SwitzerlandManufactured for: VYNE Pharmaceuticals Inc., Bridgewater, NJ 08807
Product of Portugal or Switzerland
103G1002
ZILXI is a registered trademark of an affiliate of VYNE Therapeutics Inc.All other trademarks are the properties of their respective owners.
Copyright © 2020, VYNE Therapeutics Inc. All rights reserved.
For more information, go to www.ZILXI.com or call VYNE Pharmaceuticals Inc. at 1-844-375-3673.
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 01/2021
Instructions for Use
ZILXI® (ZILK-see)
(minocycline)
topical foamImportant Information: ZILXI is for use on skin only (topical use). ZILXI is not for use in your mouth, eyes or vagina.
Read this Instructions for Use before you start using ZILXI and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. Use ZILXI exactly as your healthcare provider tells you.
Before applying ZILXI:
- When using a new ZILXI can, wait until the can has warmed to room temperature before use.
- If necessary, wash your face gently with a mild non-medicated cleanser, rinse with water, and pat your skin dry.
- Apply ZILXI to your entire face at about the same time each day, at least 1 hour before bedtime.
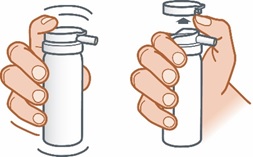
Step 1: Shake the can well. Place thumb under tab above nozzle and lift up to remove the cap from the ZILXI can.
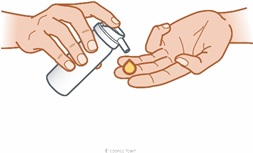
Step 2: Press the top of the can to dispense a small amount (cherry-sized amount) of ZILXI onto your fingertips.
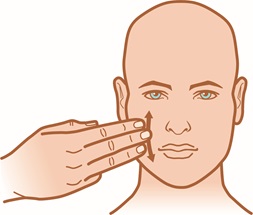
Step 3: Apply a thin layer of ZILXI and gently rub onto your entire face. You may need to use additional ZILXI to make sure that your entire face is treated.
Wash your hands after applying ZILXI.ZILXI can stain fabric. This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 01/2021
Manufactured by: ASM Aerosol-Service AG, Mohlin, Switzerland
Manufactured for: VYNE Pharmaceuticals Inc., Bridgewater, NJ 08807
Product of Portugal or Switzerland103G2002
ZILXI is a registered trademark of an affiliate of VYNE Therapeutics Inc.
All other trademarks are the properties of their respective owners.
Copyright © 2020, VYNE Therapeutics Inc. All rights reserved. -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
ZILXI Trade Labels for Country-of-Origin Portugal
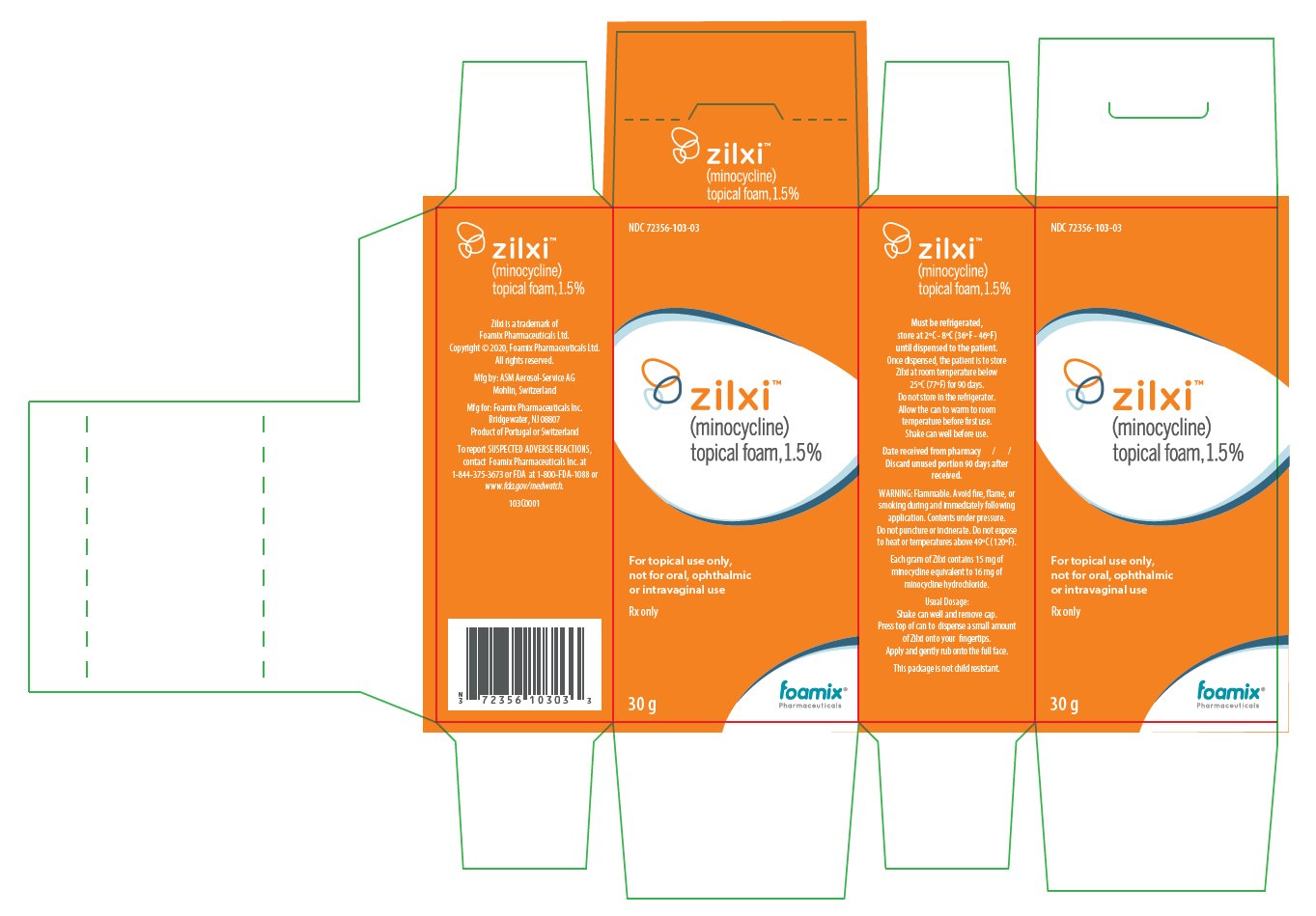
NDC: 72356-103-03
zilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
30 g

NDC: 72356-103-03
zilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
30 g
ZILXI Physician Sample Labels for Country-of-Origin Portugal
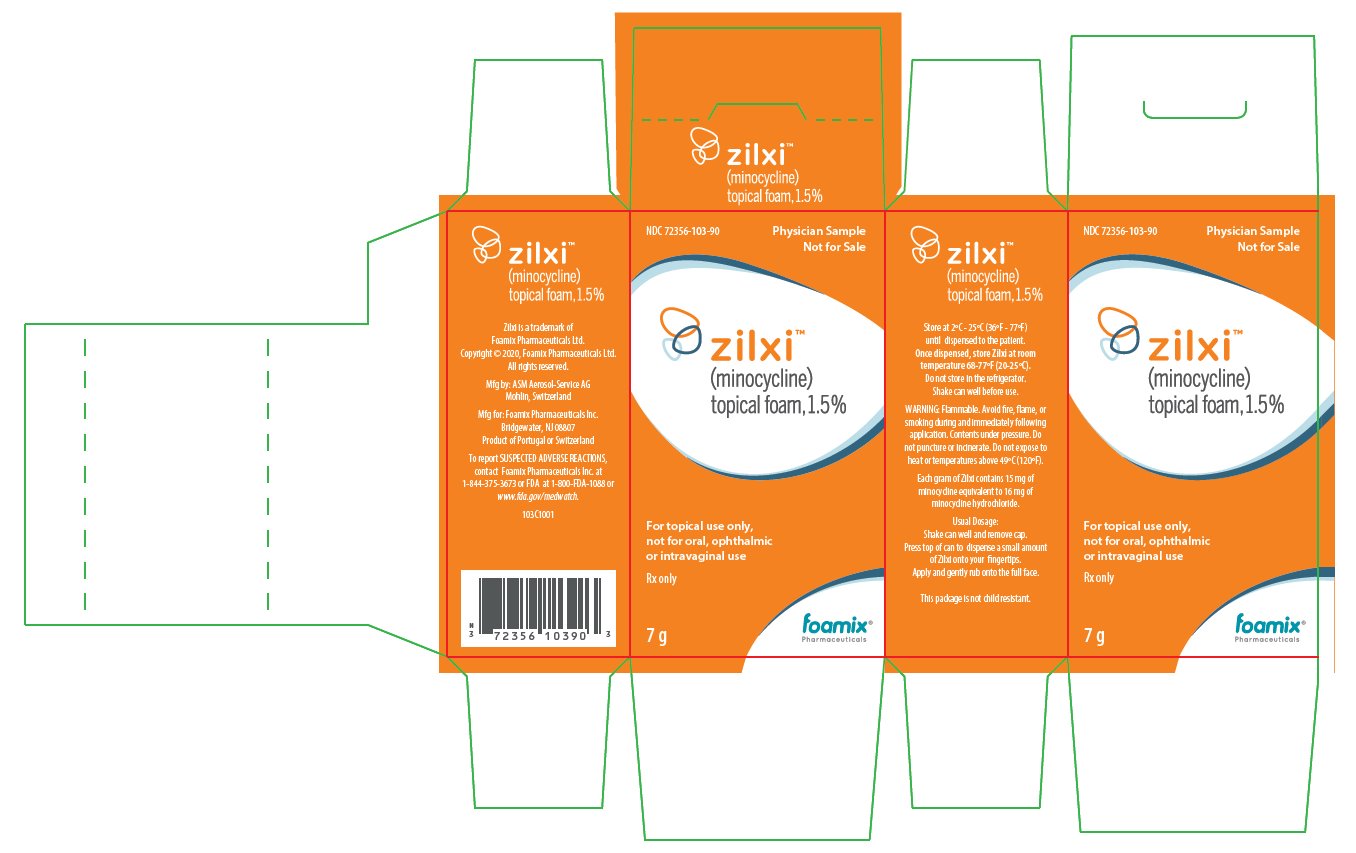
NDC: 72356-103-90
Physician Sample
Not for Salezilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
7 g

NDC: 72356-103-90
Physician Sample
Not for Salezilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
7 g
ZILXI Trade Labels for Country-of-Origin Switzerland
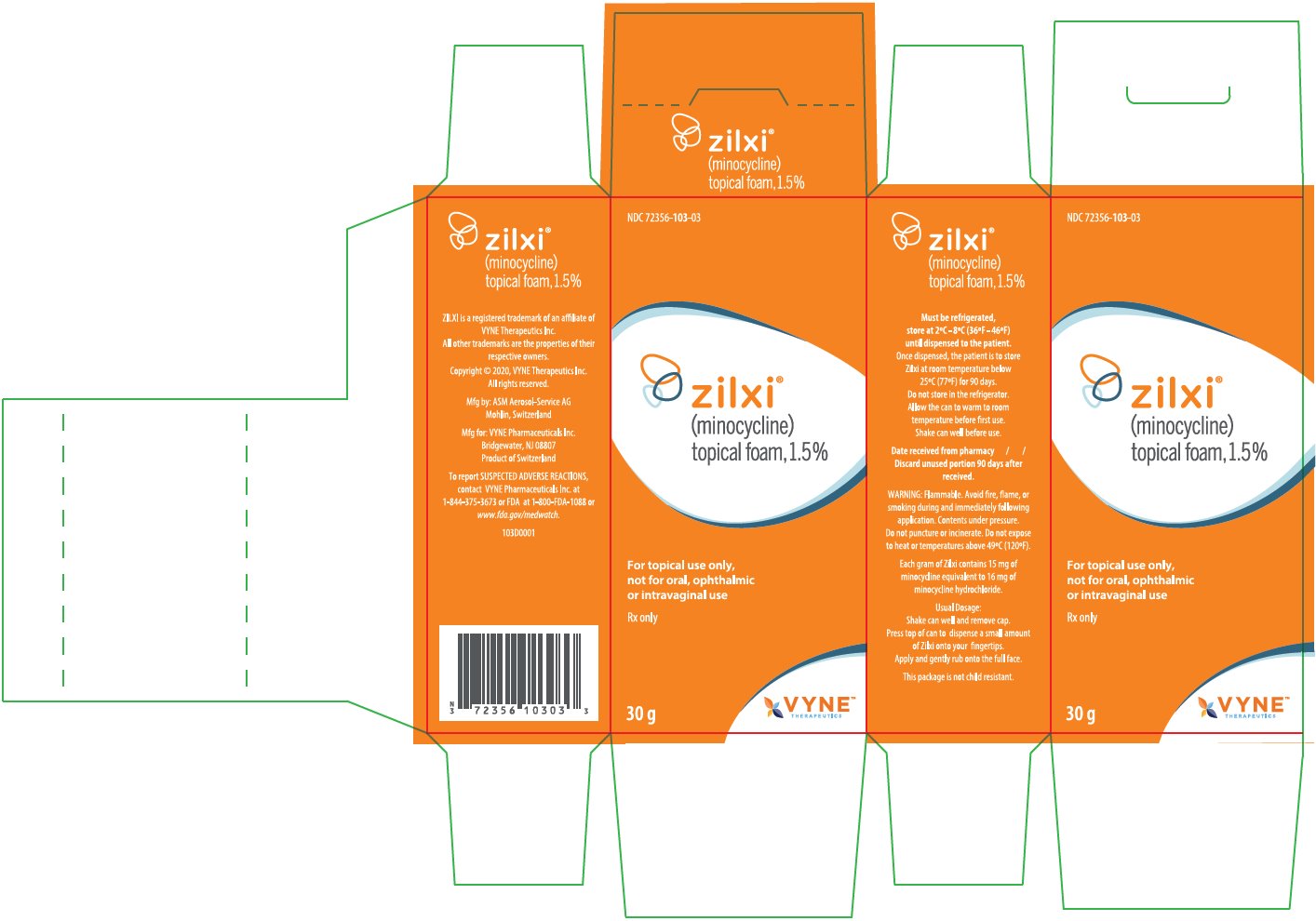
NDC: 72356-103-03
zilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
30 g

NDC: 72356-103-03
zilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
30 g
ZILXI Physician Sample Labels for Country-of-Origin Switzerland
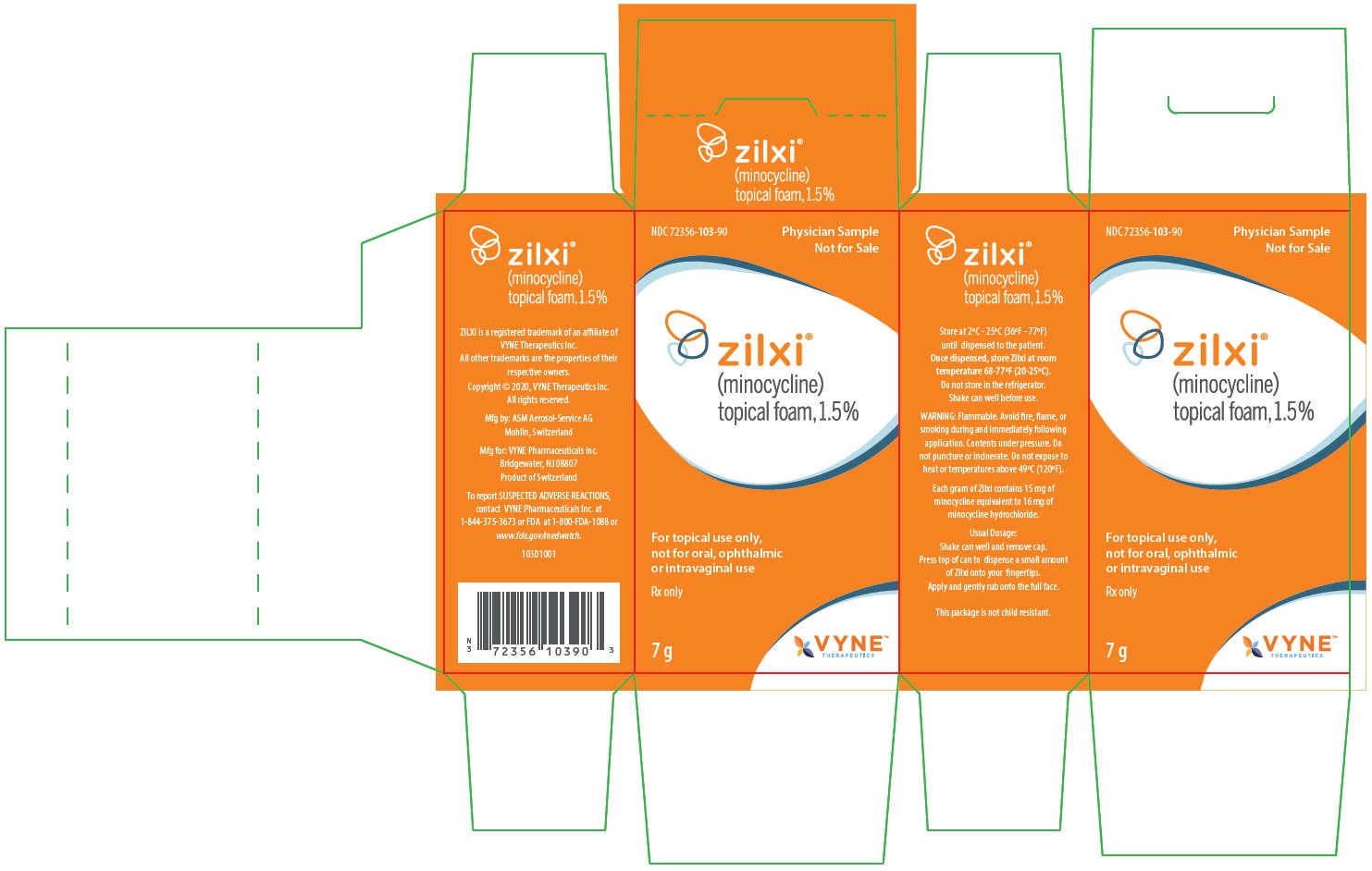
NDC: 72356-103-90
Physician Sample
Not for Salezilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
7 g

NDC: 72356-103-90
Physician Sample
Not for Salezilxi®
(minocycline)
topical foam, 1.5%For topical use only, not for oral, ophthalmic or intravaginal use
Rx only
7 g
-
INGREDIENTS AND APPEARANCE
ZILXI
minocycline aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72356-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOCYCLINE HYDROCHLORIDE (UNII: 0020414E5U) (MINOCYCLINE - UNII:FYY3R43WGO) MINOCYCLINE 15 mg in 1 g Inactive Ingredients Ingredient Name Strength SOYBEAN OIL (UNII: 241ATL177A) COCONUT OIL (UNII: Q9L0O73W7L) LIGHT MINERAL OIL (UNII: N6K5787QVP) CYCLOMETHICONE (UNII: NMQ347994Z) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARIC ACID (UNII: 4ELV7Z65AP) MYRISTYL ALCOHOL (UNII: V42034O9PU) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WHITE WAX (UNII: 7G1J5DA97F) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) DOCOSANOL (UNII: 9G1OE216XY) BUTANE (UNII: 6LV4FOR43R) ISOBUTANE (UNII: BXR49TP611) PROPANE (UNII: T75W9911L6) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72356-103-03 1 in 1 CARTON 10/01/2020 12/31/2024 1 30 g in 1 CAN; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 72356-103-90 1 in 1 CARTON 10/01/2020 07/31/2022 2 7 g in 1 CAN; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213690 10/01/2020 Labeler - Journey Medical Corporation (079640860) Establishment Name Address ID/FEI Business Operations ASM Aerosol-Service AG 480286111 manufacture(72356-103)
Trademark Results [ZILXI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZILXI 88758482 not registered Live/Pending |
Foamix Pharmaceuticals Ltd 2020-01-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.