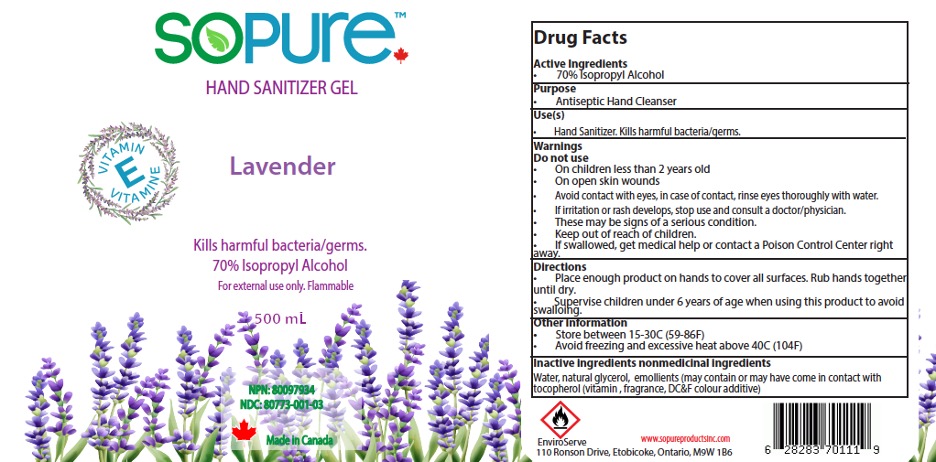
SoPure Hand Sanitizer 70% IPA Gel
SoPure Hand Sanitizer Gel by
Drug Labeling and Warnings
SoPure Hand Sanitizer Gel by is a Otc medication manufactured, distributed, or labeled by EnviroServe Chemicals & Cleaners Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SOPURE HAND SANITIZER GEL- isopropyl alcohol 70% gel hand sanitizer gel
EnviroServe Chemicals & Cleaners Ltd
----------
SoPure Hand Sanitizer 70% IPA Gel
Warnings
- Flamable. Keep away from fire or flame
- For external use only
- When using this product avoid contact with eyes. In case of eye contact, flush eyes with water
- Stop use and ask a doctor if irritation or rash appears and lasts
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
When using
When using this product keep out of eyes, ears and mouth. In case of contact with eyes, rinse, stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center.
Stop use section
Stop use and ask a doctor if irritation or rash occurs. These may be a sign of a serious condition.
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center.
Directions
- Dispense enough product in your palm to cover hands and rub hands together briskly until dry
- Children under 6 years of age should be supervised when using this product
| SOPURE HAND SANITIZER GEL
isopropyl alcohol 70% gel hand sanitizer gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - EnviroServe Chemicals & Cleaners Ltd (243690711) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EnviroServe Chemicals & Cleaners Ltd | 243690711 | manufacture(80773-004) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.