10% GLUCOSE INJECTION
Glucose by
Drug Labeling and Warnings
Glucose by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter Healthcare (Shanghai) Co. Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GLUCOSE- dextrose anhydrous injection, solution
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
10% GLUCOSE INJECTION
HEALTH CARE PROFESSIONAL LETTER
Reporting Adverse Events or Product Quality Issues
To report adverse events associated with these imported products, please call Baxter at 1-866-888-2472, or fax: 1- 800-759-1801. Adverse events or quality problems experienced with the use of these imported products may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
To report product quality issues associated with these imported products, please contact Baxter Product Surveillance through Baxter - Product Feedback Portal (https://productfeedback.baxter.com/).
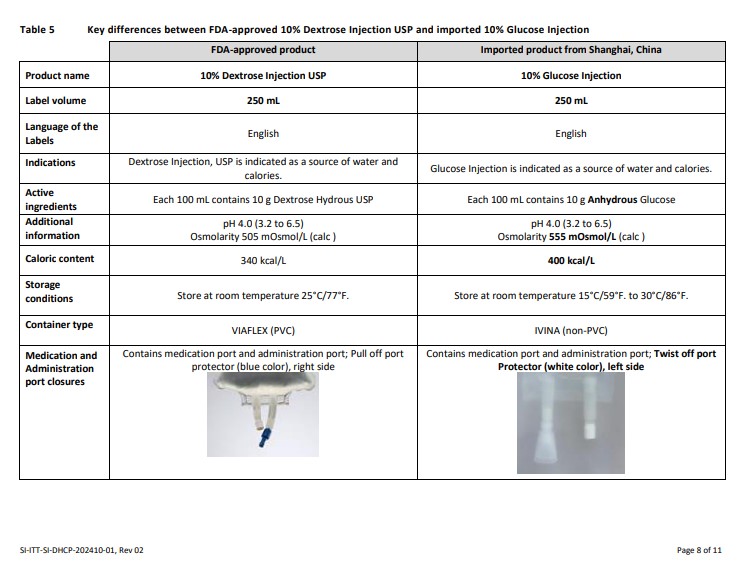
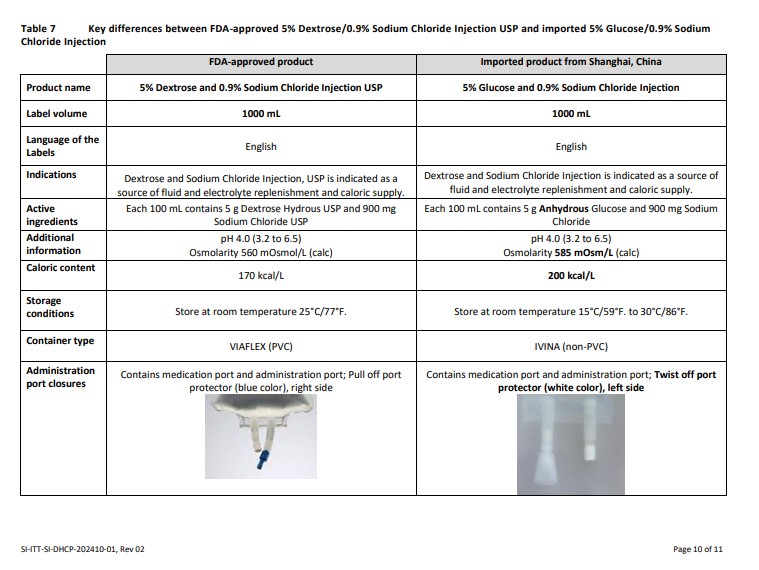
Please also refer to the local prescribing information of the imported product, translated into English, available for:
0.9% Sodium Chloride Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723233)
5% Glucose Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723235)
10% Glucose Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723237)
5% Glucose/0.9% Sodium Chloride Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723238)
Please refer to the FDA-approved prescribing information for each drug product listed below:
0.9% Sodium Chloride Injection USP (click https://www.fda.report/dailymed/getFile.cfm?setid=f55bd888-5e01-474d-871b-24654c070178&type=pdf/f55bd888-5e01-474d-871b-24654c070178)
5% Dextrose Injection USP (click https://www.fda.report/dailymed/getFile.cfm?setid=3bb406a9-f5cb-403a-b1bb-5c4facbea3d5&type=pdf/3bb406a9-f5cb-403a-b1bb-5c4facbea3d5)
10% Dextrose Injection USP (click https://www.fda.report/dailymed/getFile.cfm?setid=3bb406a9-f5cb-403a-b1bb-5c4facbea3d5&type=pdf/3bb406a9-f5cb-403a-b1bb-5c4facbea3d5)
5% Dextrose/0.9% Sodium Chloride Injection USP (click https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/016678s007,016683s103,016687s104,016689s107,016697s098lbl.pdf)
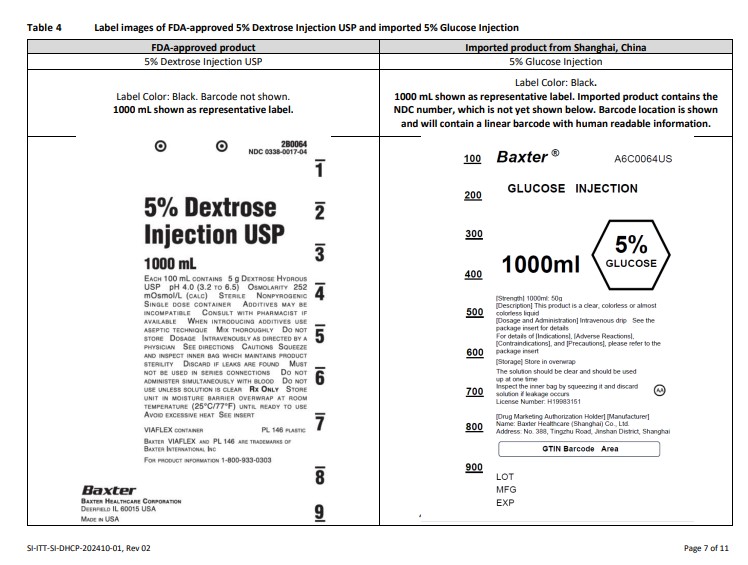
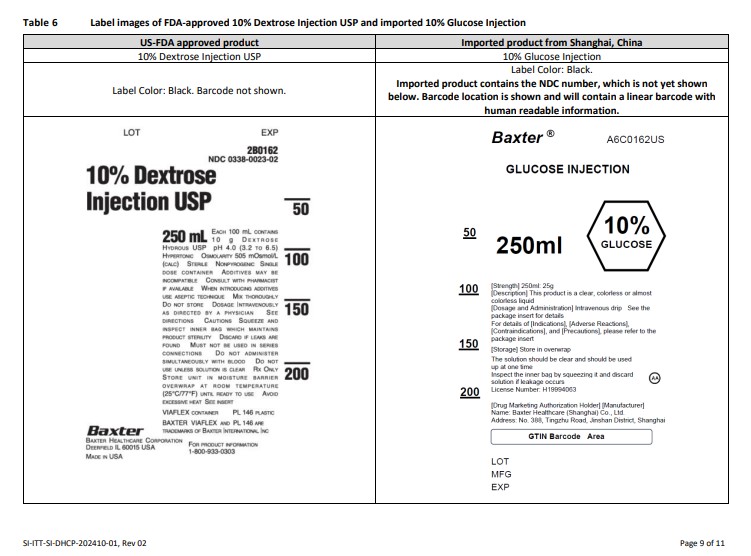
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Baxter Logo Trademark
A6C0162US
GLUCOSE INJECTION
50
100
150
200
250ml
10% GLUCOSE
[Strength] 250ml: 25g
[Description] This product is a colorless or almost
colorless clear liquid
[Dosage and Administration] Intravenous drip See the
package insert for details
For details of [Indications], [Adverse Reactions],
[Contraindications], and [Precautions], please refer to the
package insert
[Storage] Store in overwrap
The solution should be clear and should be used
up at one time
Inspect the inner bag by squeezing it and discard
solution if leakage occurs
License Number: H19994063
AA
[Drug Marketing Authorization Holder] [Manufacturer]
Name: Baxter Healthcare (Shanghai) Co., Ltd.
Address: No. 388, Tingzhu Road, Jinshan District, Shanghai
BarCode
(01) 00303389797012
LOT
MFG
EXP
10% Glucose Injection
250ml X 40
LOT S0000000 EXP YYYY-MM
A6C0162US 1C/N LIC H19994063
10% Glucose Injection
250ml X 40
LOT S0000000 EXP YYYY-MM
MFG YYYY-MM-DD 1C/N 0000
| GLUCOSE
dextrose anhydrous injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare (Shanghai) Co. Ltd. | 527191860 | MANUFACTURE(0338-9797) , ANALYSIS(0338-9797) , LABEL(0338-9797) , PACK(0338-9797) , STERILIZE(0338-9797) | |
Trademark Results [Glucose]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GLUCOSE 78764828 not registered Dead/Abandoned |
Glucose Media, Inc. 2005-12-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.