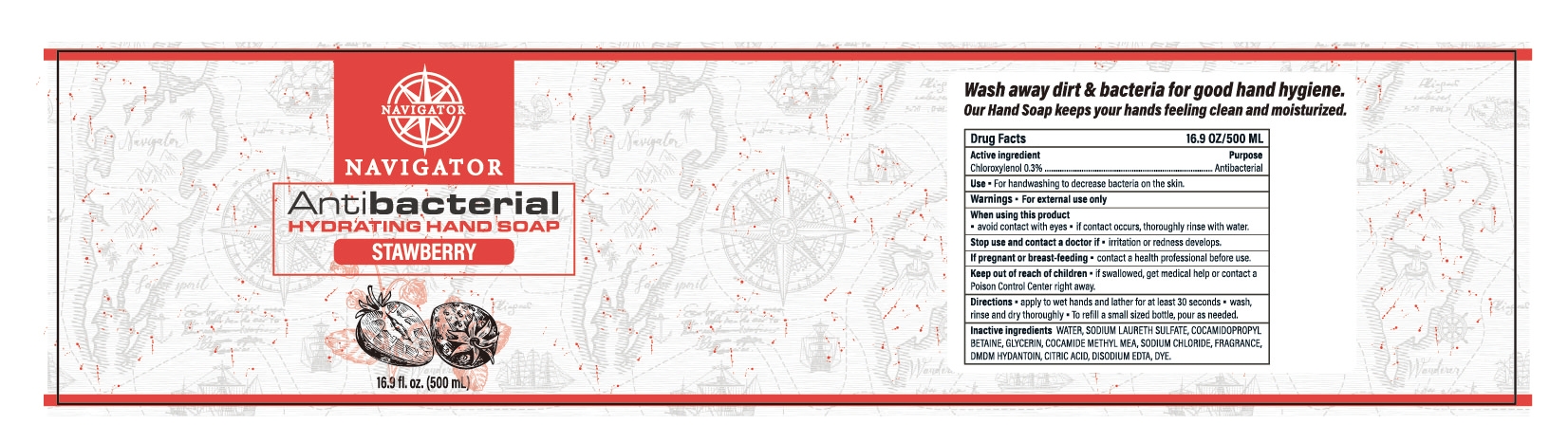
HANDSOAP by Suzhou Longlifu Health Food Co.,Ltd. 71333-105
HANDSOAP by
Drug Labeling and Warnings
HANDSOAP by is a Otc medication manufactured, distributed, or labeled by Suzhou Longlifu Health Food Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HANDSOAP- chloroxylenol gel
Suzhou Longlifu Health Food Co.,Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
71333-105
Keep out of reach of children
- if swallowed,get medical help or contact a Poison Control Center right away.
Directions:
- apply to wet hands and lather for at least 30 seconds.
- wash,rinse and dry thoroughly
- To refill a small sized bottle,pour as needed.
| HANDSOAP
chloroxylenol gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Suzhou Longlifu Health Food Co.,Ltd. (530536671) |
| Registrant - Suzhou Longlifu Health Food Co.,Ltd. (530536671) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Suzhou Longlifu Health Food Co.,Ltd. | 530536671 | manufacture(71333-105) | |
Revised: 10/2022
Document Id: ea1dcf35-4bb5-14b7-e053-2a95a90a9140
Set id: 11bad576-2db2-4274-98bf-fddeb5a90b04
Version: 2
Effective Time: 20221003