BRIVARACETAM tablet, film coated
brivaracetam by
Drug Labeling and Warnings
brivaracetam by is a Prescription medication manufactured, distributed, or labeled by Novadoz Pharmaceuticals LLC, MSN LABORATORIES PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BRIVARACETAM TABLETS safely and effectively. See full prescribing information for BRIVARACETAM TABLETS.
BRIVARACETAM tablets, for oral use, CV
Initial U.S. Approval: 2016RECENT MAJOR CHANGES
Warnings and Precautions (5.5) 8/2025
INDICATIONS AND USAGE
Brivaracetam tablets are indicated for the treatment of partial-onset seizures in patients 1 month of age and older. (1)
DOSAGE AND ADMINISTRATION
- Adults (16 Years and Older): The recommended starting dosage for monotherapy or adjunctive therapy is 50 mg twice daily (100 mg per day). Based on individual patient tolerability and therapeutic response, the dosage may be adjusted down to 25 mg twice daily (50 mg per day) or up to 100 mg twice daily (200 mg per day). (2.1)
- Pediatric Patients (1 Month to less than 16 Years): The recommended dosage is based on body weight and is administered orally twice daily (2.1)
- Hepatic Impairment: Dose adjustment is recommended for all stages of hepatic impairment. (2.5)
DOSAGE FORMS AND STRENGTHS
- Tablets: 10 mg, 25 mg, 50 mg, 75 mg, and 100 mg (3)
CONTRAINDICATIONS
Hypersensitivity to brivaracetam or any of the inactive ingredients in brivaracetam. (4)
WARNINGS AND PRECAUTIONS
- Suicidal Behavior and Ideation: Monitor patients for suicidal behavior and ideation. (5.1)
- Neurological Adverse Reactions: Monitor for somnolence and fatigue, and advise patients not to drive or operate machinery until they have gained sufficient experience on brivaracetam. (5.2)
- Psychiatric Adverse Reactions: Behavioral reactions including psychotic symptoms, irritability, depression, aggressive behavior, and anxiety; monitor patients for symptoms. (5.3)
- Hypersensitivity: Bronchospasm and Angioedema: Advise patients to seek immediate medical care. Discontinue and do not restart brivaracetam if hypersensitivity occurs. (5.4)
- Serious Dermatologic Reactions: Discontinue brivaracetam unless an alternative etiology is established (5.5)
- Withdrawal of Antiepileptic Drugs: Brivaracetam should be gradually withdrawn. (5.6)
ADVERSE REACTIONS
Adults: Most common adverse reactions (at least 5% for brivaracetam and at least 2% more frequently than placebo) are somnolence/sedation, dizziness, fatigue, and nausea/vomiting. (6.1)
Pediatric Patients: Most common adverse reactions are similar to those seen in adult patients. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novadoz Pharmaceuticals LLC at 1-855-668-2369 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Rifampin: Because of decreased concentrations, increasing brivaracetam dosage in patients on concomitant rifampin is recommended. (2.6, 7.1)
- Carbamazepine: Because of increased exposure to carbamazepine metabolite, if tolerability issues arise, consider reducing carbamazepine dosage in patients on concomitant brivaracetam. (7.2)
- Phenytoin: Because phenytoin concentrations can increase, phenytoin levels should be monitored in patients on concomitant brivaracetam. (7.3)
- Levetiracetam: Brivaracetam had no added therapeutic benefit when co-administered with levetiracetam. (7.4)
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
2.2 Administration Instructions for Brivaracetam Tablets
2.4 Discontinuation of Brivaracetam Tablets
2.5 Patients with Hepatic Impairment
2.6 Co-administration with Rifampin
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Behavior and Ideation
5.2 Neurological Adverse Reactions
5.3 Psychiatric Adverse Reactions
5.4 Hypersensitivity: Bronchospasm and Angioedema
5.5 Serious Dermatologic Reactions
5.6 Withdrawal of Antiepileptic Drugs
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Rifampin
7.2 Carbamazepine
7.3 Phenytoin
7.4 Levetiracetam
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
Monotherapy or Adjunctive Therapy
The recommended dosage for patients 1 month of age and older is included in Table 1. In pediatric patients weighing less than 50 kg, the recommended dosing regimen is dependent upon body weight. When initiating treatment, gradual dose escalation is not required. Dosage should be adjusted based on clinical response and tolerability.
Table 1: Recommended Dosage for Patients 1 Month of Age and OlderAge and Body Weight
Initial Dosage
Minimum and Maximum Maintenance Dosage
Adults (16 years and older)
50 mg twice daily
(100 mg per day)
25 mg to 100 mg twice daily
(50 mg to 200 mg per day)
Pediatric patients weighing 50 kg or more
25 mg to 50 mg twice daily
(50 mg to 100 mg per day)
25 mg to 100 mg twice daily
(50 mg to 200 mg per day)
Pediatric patients weighing 20 kg to less than 50 kg
0.5 mg/kg to 1 mg/kg twice daily
(1 mg/kg to 2 mg/kg per day)
0.5 mg/kg to 2 mg/kg twice daily
(1 mg/kg to 4 mg/kg per day)
Pediatric patients weighing 11 kg to less than 20 kg
0.5 mg/kg to 1.25 mg/kg twice daily
(1 mg/kg to 2.5 mg/kg per day)
0.5 mg/kg to 2.5 mg/kg twice daily
(1 mg/kg to 5 mg/kg per day)
Pediatric patients
weighing less than 11 kg
0.75 mg/kg to 1.5 mg/kg twice daily
(1.5 mg/kg to 3 mg/kg per day)
0.75 mg/kg to 3 mg/kg twice daily
(1.5 mg/kg to 6 mg/kg per day)
2.2 Administration Instructions for Brivaracetam Tablets
Brivaracetam tablets can be initiated with oral administration.
Brivaracetam tablets may be taken with or without food.
Brivaracetam Tablets
Brivaracetam tablets should be swallowed whole with liquid. Brivaracetam tablets should not be chewed or crushed.
2.4 Discontinuation of Brivaracetam Tablets
Avoid abrupt withdrawal from brivaracetam tablets in order to minimize the risk of increased seizure frequency and status epilepticus [see Warnings and Precautions (5.6) and Clinical Studies (14)].
2.5 Patients with Hepatic Impairment
The recommended dosage for patients with hepatic impairment is included in Table 2 [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Table 2: Recommended Dosage for Patients with Hepatic Impairment
Age and Body
Weight
Initial Dosage
Maximum Maintenance Dosage
Adults (16 years and older)
25 mg twice daily
(50 mg per day)
75 mg twice daily
(150 mg per day)
Pediatric patients weighing
50 kg or more
Pediatric patients weighing 20 kg to less than 50 kg
0.5 mg/kg twice daily
(1 mg/kg per day)
1.5 mg/kg twice daily
(3 mg/kg per day)
Pediatric patients weighing 11 kg to less than 20 kg
0.5 mg/kg twice daily
(1 mg/kg per day)
2 mg/kg twice daily
(4 mg/kg per day)
Pediatric patients
weighing less than 11 kg
0.75 mg/kg twice daily
(1.5 mg/kg per day)
2.25 mg/kg twice daily
(4.5 mg/kg per day)
2.6 Co-administration with Rifampin
Increase the brivaracetam tablets dosage in patients on concomitant rifampin by up to 100% (i.e., double the dosage) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
Tablets
- 10 mg: White to off white round, film-coated tablet debossed with “B10” on one side and “M” on other side, free from physical defects.
- 25 mg: Pink, Oval, film-coated tablet debossed with “B11” on one side and “M” on other side, free from physical defects.
- 50 mg: Yellow, Oval, film-coated tablet debossed with “B12” on one side and “M” on other side, free from physical defects.
- 75 mg: Purple, Oval, film-coated tablet debossed with “B13” on one side and “M” on other side, free from physical defects.
- 100 mg: Orange, Oval, film-coated tablet debossed with “B14” on one side and “M” on other side, free from physical defects.
-
4 CONTRAINDICATIONS
Hypersensitivity to brivaracetam or any of the inactive ingredients in brivaracetam tablets (bronchospasm and angioedema have occurred) [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including brivaracetam, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Table 3: Risk of Suicidal Thoughts or Behaviors by Indication for Antiepileptic Drugs in the Pooled Analysis
Indication
Placebo Patients with Events Per 1000 Patients
Drug Patients with Events Per 1000 Patients
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients
Risk Difference: Additional Drug Patients with Events Per 1000 Patients
Epilepsy
1.0
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1.0
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials in patients with epilepsy than in clinical trials in patients with psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing brivaracetam or any other AED must balance the risk of suicidal thoughts or behaviors with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.5.2 Neurological Adverse Reactions
Brivaracetam causes somnolence, fatigue, dizziness, and disturbance in coordination. Patients should be monitored for these signs and symptoms and advised not to drive or operate machinery until they have gained sufficient experience on brivaracetam to gauge whether it adversely affects their ability to drive or operate machinery.
Somnolence and Fatigue
Brivaracetam causes dose-dependent increases in somnolence and fatigue-related adverse reactions (fatigue, asthenia, malaise, hypersomnia, sedation, and lethargy) [see Adverse Reactions (6.1)]. In the Phase 3 controlled adjunctive epilepsy trials, these events were reported in 25% of patients randomized to receive brivaracetam at least 50 mg/day (20% at 50 mg/day, 26% at 100 mg/day, and 27% at 200 mg/day) compared to 14% of patients who received placebo. The risk is greatest early in treatment but can occur at any time.
Dizziness and Disturbance in Gait and Coordination
Brivaracetam causes adverse reactions related to dizziness and disturbance in gait and coordination (dizziness, vertigo, balance disorder, ataxia, nystagmus, gait disturbance, and abnormal coordination) [see Adverse Reactions (6.1)]. In the Phase 3 controlled adjunctive epilepsy trials, these events were reported in 16% of patients randomized to receive brivaracetam at least 50 mg/day compared to 10% of patients who received placebo. The risk is greatest early in treatment but can occur at any time.
5.3 Psychiatric Adverse Reactions
Brivaracetam causes psychiatric adverse reactions. In the Phase 3 controlled adjunctive epilepsy trials, psychiatric adverse reactions were reported in approximately 13% of patients who received brivaracetam (at least 50 mg/day) compared to 8% of patients who received placebo. Psychiatric events included both non-psychotic symptoms (irritability, anxiety, nervousness, aggression, belligerence, anger, agitation, restlessness, depression, depressed mood, tearfulness, apathy, altered mood, mood swings, affect lability, psychomotor hyperactivity, abnormal behavior, and adjustment disorder) and psychotic symptoms (psychotic disorder along with hallucination, paranoia, acute psychosis, and psychotic behavior). A total of 1.7% of adult patients treated with brivaracetam discontinued treatment because of psychiatric reactions compared to 1.3% of patients who received placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults [see Adverse Reactions (6.1)and Use in Specific Populations (8.4)].
5.4 Hypersensitivity: Bronchospasm and Angioedema
Brivaracetam can cause hypersensitivity reactions. Bronchospasm and angioedema have been reported in patients taking brivaracetam. If a patient develops hypersensitivity reactions after treatment with brivaracetam, the drug should be discontinued. Brivaracetam is contraindicated in patients with a prior hypersensitivity reaction to brivaracetam or any of the inactive ingredients [see Contraindications (4)].
5.5 Serious Dermatologic Reactions
Serious dermatologic reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in patients treated with brivaracetam. Time to onset of the serious dermatologic reaction ranged from 3 to 45 days after brivaracetam initiation in reported cases. Brivaracetam should be discontinued at the first sign of a rash, unless the rash is clearly not drug-related. If signs or symptoms suggest a serious dermatologic reaction, use of brivaracetam should not be resumed and alternative therapy should be considered.
5.6 Withdrawal of Antiepileptic Drugs
As with most antiepileptic drugs, brivaracetam should generally be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus [see Dosage and Administration (2.4) and Clinical Studies (14)]. But if withdrawal is needed because of a serious adverse event, rapid discontinuation can be considered.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.1)]
- Neurological Adverse Reactions[see Warnings and Precautions (5.2)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.3)]
- Hypersensitivity: Bronchospasm and Angioedema [see Warnings and Precautions (5.4)]
- Serious Dermatologic Reactions [see Warnings and Precautions (5.5)]
- Withdrawal of Antiepileptic Drugs [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In all controlled and uncontrolled trials performed in adult epilepsy patients, brivaracetam was administered as adjunctive therapy to 2437 patients. Of these patients, 1929 were treated for at least 6 months, 1500 for at least 12 months, 1056 for at least 24 months, and 758 for at least 36 months. A total of 1558 patients (1099 patients treated with brivaracetam and 459 patients treated with placebo) constituted the safety population in the pooled analysis of Phase 3 placebo-controlled studies in patients with partial-onset seizures (Studies 1, 2, and 3) [see Clinical Studies (14)]. The adverse reactions presented in Table 4 are based on this safety population; the median length of treatment in these studies was 12 weeks. Of the patients in those studies, approximately 51% were male, 74% were Caucasian, and the mean age was 38 years.
In the Phase 3 controlled epilepsy studies, adverse events occurred in 68% of patients treated with brivaracetam and 62% treated with placebo. The most common adverse reactions occurring at a frequency of at least 5% in patients treated with brivaracetam doses of at least 50 mg/day and greater than placebo were somnolence and sedation (16%), dizziness (12%), fatigue (9%), and nausea and vomiting symptoms (5%).
The discontinuation rates due to adverse events were 5%, 8%, and 7% for patients randomized to receive brivaracetam at the recommended doses of 50 mg, 100 mg, and 200 mg/day, respectively, compared to 4% in patients randomized to receive placebo.
Table 4 lists adverse reactions for brivaracetam that occurred at least 2% more frequently for brivaracetam doses of at least 50 mg/day than placebo.
Table 4: Adverse Reactions in Pooled Placebo-Controlled Adjunctive Therapy Studies in Adult Patients with Partial-Onset Seizures (Brivaracetam 50 mg/day, 100 mg/day, and 200 mg/day)
Adverse Reactions
Brivaracetam (N=803)
%
Placebo (N=459)
%
Gastrointestinal disorders
Nausea/vomiting symptoms
5
3
Constipation
2
0
Nervous system disorders
Somnolence and sedation
16
8
Dizziness
12
7
Fatigue
9
4
Cerebellar coordination and balance disturbances*
3
1
Psychiatric disorders
Irritability
3
1
* Cerebellar coordination and balance disturbances includes ataxia, balance disorder, coordination abnormal, and nystagmus.
There was no apparent dose-dependent increase in adverse reactions listed in Table 4 with the exception of somnolence and sedation.
Pediatric Patients
Safety of brivaracetam was evaluated in two open-label, safety and pharmacokinetic trials in pediatric patients 2 months to less than 16 years of age. Across studies of pediatric patients with partial onset seizures, 186 patients received brivaracetam oral solution or tablet, of whom 123 received brivaracetam for at least 12 months. Adverse reactions reported in clinical studies of pediatric patients were generally similar to those seen in adult patients. Decreased appetite was also observed in these pediatric trials.
Hematologic Abnormalities
Brivaracetam can cause hematologic abnormalities. In the Phase 3 controlled adjunctive epilepsy studies, a total of 1.8% of brivaracetam-treated patients and 1.1% of placebo-treated patients had at least one clinically significant decreased white blood cell count (<3.0 x 109/L), and 0.3% of brivaracetam-treated patients and 0% of placebo-treated patients had at least one clinically significant decreased neutrophil count (<1.0 x 109/L).Comparison by Sex
There were no significant differences by sex in the incidence of adverse reactions.6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of brivaracetam. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Serious dermatologic reactions (e.g., Stevens-Johnson syndrome and toxic epidermal necrolysis) [see Warnings and Precautions (5.5)]
-
7 DRUG INTERACTIONS
7.1 Rifampin
Co-administration with rifampin decreases brivaracetam plasma concentrations likely because of CYP2C19 induction [see Clinical Pharmacology (12.3)]. Prescribers should increase the brivaracetam dose by up to 100% (i.e., double the dosage) in patients while receiving concomitant treatment with rifampin [see Dosage and Administration (2.6)].
7.2 Carbamazepine
Co-administration with carbamazepine may increase exposure to carbamazepine-epoxide, the active metabolite of carbamazepine. Though available data did not reveal any safety concerns, if tolerability issues arise when co-administered, carbamazepine dose reduction should be considered [see Clinical Pharmacology (12.3)].
7.3 Phenytoin
Because brivaracetam can increase plasma concentrations of phenytoin, phenytoin levels should be monitored in patients when concomitant brivaracetam is added to or discontinued from ongoing phenytoin therapy [see Clinical Pharmacology (12.3)].
7.4 Levetiracetam
Brivaracetam provided no added therapeutic benefit to levetiracetam when the two drugs were co-administered[see Clinical Studies (14)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as brivaracetam, during pregnancy. Encourage patients who are taking brivaracetam during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling the toll free number 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
Available data from the North American Antiepileptic Drug (NAAED) pregnancy registry, a prospective cohort study, case reports, and a case series are insufficient to identify a risk of major birth defects, miscarriage or other maternal or fetal outcomes associated with brivaracetam use during pregnancy. In animal studies, brivaracetam produced evidence of developmental toxicity (increased embryofetal mortality and decreased fetal body weights in rabbits; decreased growth, delayed sexual maturation, and long-term neurobehavioral changes in rat offspring) at maternal plasma exposures greater than clinical exposures [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Oral administration of brivaracetam (0, 150, 300, or 600 mg/kg/day) to pregnant rats during the period of organogenesis did not produce any significant maternal or embryofetal toxicity. The highest dose tested was associated with maternal plasma exposures (AUC) approximately 30 times exposures in humans at the maximum recommended dose (MRD) of 200 mg/day.
Oral administration of brivaracetam (0, 30, 60, 120, or 240 mg/kg/day) to pregnant rabbits during the period of organogenesis resulted in embryofetal mortality and decreased fetal body weights at the highest dose tested, which was also maternally toxic. The highest no-effect dose (120 mg/kg/day) was associated with maternal plasma exposures approximately 4 times human exposures at the MRD.
When brivaracetam (0, 150, 300, or 600 mg/kg/day) was orally administered to rats throughout pregnancy and lactation, decreased growth, delayed sexual maturation (female), and long-term neurobehavioral changes were observed in the offspring at the highest dose. The highest no-effect dose (300 mg/kg/day) was associated with maternal plasma exposures approximately 7 times human exposures at the MRD.
Brivaracetam was shown to readily cross the placenta in pregnant rats after a single oral (5 mg/kg) dose of 14C-brivaracetam. From 1 hour post dose, radioactivity levels in fetuses, amniotic fluid, and placenta were similar to those measured in maternal blood.8.2 Lactation
Risk Summary
Data from published literature indicate that brivaracetam is present in human milk. There is insufficient information on the effects of brivaracetam on the breastfed infant or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for brivaracetam and any potential adverse effects on the breastfed infant from brivaracetam or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of brivaracetam have been established in pediatric patients 1 month to less than 16 years of age. Use of brivaracetam in these age groups is supported by evidence from adequate and well-controlled studies of brivaracetam in adults with partial-onset seizures, pharmacokinetic data from adult and pediatric patients, and safety data in pediatric patients 2 months to less than 16 years of age [see Dosage and Administration (2.1), Warnings and Precautions (5.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Safety and effectiveness in pediatric patients below the age of 1 month have not been established.
Juvenile Animal Toxicity Data
The potential adverse effects of brivaracetam on postnatal growth and development were investigated in juvenile rats and dogs. Oral administration (0, 150, 300, or 600 mg/kg/day) to rats during the neonatal and juvenile periods of development (approximately equivalent to neonatal through adolescent development in humans) resulted in increased mortality, decreased body weight gain, delayed male sexual maturation, and adverse neurobehavioral effects at the highest dose tested and decreased brain size and weight at all doses. Therefore, a no-effect dose was not established; the lowest dose tested in juvenile rats was associated with plasma exposures (AUC) approximately 2 times those in children and adolescents at the recommended maintenance dose. In dogs, oral administration (0, 15, 30, or 100 mg/kg/day) throughout the neonatal and juvenile periods of development induced liver changes similar to those observed in adult animals at the highest dose but produced no adverse effects on growth, bone density or strength, neurological testing, or neuropathology evaluation. The overall no-effect dose (30 mg/kg/day) and the no-effect dose for adverse effects on developmental parameters (100 mg/kg/day) were associated with plasma exposures approximately equal to and 4 times, respectively, those in children and adolescents at the recommended maintenance dose.
8.5 Geriatric Use
There were insufficient numbers of patients 65 years of age and older in the double-blind, placebo-controlled epilepsy trials (n=38) to allow adequate assessment of the effectiveness of brivaracetam in this population. In general, dose selection for an elderly patient should be judicious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy[see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Dose adjustments are not required for patients with impaired renal function. There are no data in patients with end-stage renal disease undergoing dialysis, and use of brivaracetam is not recommended in this patient population [see Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Brivaracetam tablets contains brivaracetam and is listed as a Schedule V controlled substance.
9.2 Abuse
In a human abuse potential study, single doses of brivaracetam at therapeutic and supratherapeutic doses were compared to alprazolam (C-IV) (1.5 mg and 3 mg). Brivaracetam at the recommended single dose (50 mg) caused fewer sedative and euphoric effects than alprazolam; however, brivaracetam at supratherapeutic single doses (200 mg and 1000 mg) was similar to alprazolam on other measures of abuse.
9.3 Dependence
There was no evidence of physical dependence potential or a withdrawal syndrome with brivaracetam in a pooled review of placebo-controlled adjunctive therapy studies [see Warnings and Precautions (5.6)].
-
10 OVERDOSAGE
There is limited clinical experience with brivaracetam overdose in humans. Somnolence and dizziness were reported in a patient taking a single dose of 1400 mg (14 times the highest recommended single dose) of brivaracetam. The following adverse reactions were reported with brivaracetam overdose: vertigo, balance disorder, fatigue, nausea, diplopia, anxiety, and bradycardia. In general, the adverse reactions associated with brivaracetam overdose were consistent with the known adverse reactions.
There is no specific antidote for overdose with brivaracetam. In the event of overdose, standard medical practice for the management of any overdose should be used. An adequate airway, oxygenation, and ventilation should be ensured; monitoring of cardiac rate and rhythm and vital signs is recommended. A certified poison control center should be contacted for updated information on the management of overdose with brivaracetam. There are no data on the removal of brivaracetam using hemodialysis, but because less than 10% of brivaracetam is excreted in urine, hemodialysis is not expected to enhance brivaracetam clearance.
-
11 DESCRIPTION
The chemical name of brivaracetam is (2S)-2-[(4R)-2-oxo-4-propyltetrahydro-1H-pyrrol-1-yl] butanamide. Its molecular formula is C11H20N2O2 and its molecular weight is 212.29. The chemical structure is:
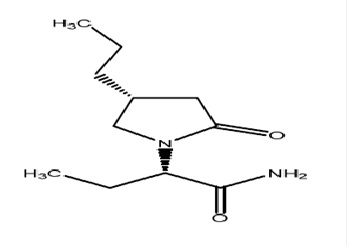
Brivaracetam is a white to off-white crystalline powder. It is very soluble in water, in buffer (pH 1.2, 4.5, and 7.4), in ethanol, in methanol, and in glacial acetic acid. It is freely soluble in acetonitrile and in acetone and soluble in toluene. It is very slightly soluble in n-hexane.
Tablets
Brivaracetam tablets are for oral administration and contain the following inactive ingredients: anhydrous lactose, croscarmellose sodium, lactose monohydrate, magnesium stearate, silicified microcrystalline cellulose and film-coating agents specified below:
10 mg tablets: titanium dioxide, polyethylene glycol, talc.
25 mg tablets: titanium dioxide, polyethylene glycol, talc, iron oxide red.
50 mg tablets: titanium dioxide, polyethylene glycol, talc, iron oxide yellow.
75 mg tablets: titanium dioxide, polyethylene glycol, talc, iron oxide red, black iron oxide.
100 mg tablets: titanium dioxide, polyethylene glycol, talc, FD&C yellow No. 6. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism by which brivaracetam exerts its anticonvulsant activity is not known. Brivaracetam displays a high and selective affinity for synaptic vesicle protein 2A (SV2A) in the brain, which may contribute to the anticonvulsant effect.
12.2 Pharmacodynamics
Interactions with Alcohol
In a pharmacokinetic and pharmacodynamic interaction study in healthy subjects, co-administration of brivaracetam (single dose 200 mg [2 times greater than the highest recommended single dose]) and ethanol (continuous intravenous infusion to achieve a blood alcohol concentration of 60 mg/100 mL during 5 hours) increased the effects of alcohol on psychomotor function, attention, and memory. Co-administration of brivaracetam and ethanol caused a larger decrease from baseline in saccadic peak velocity, smooth pursuit, adaptive tracking performance, and Visual Analog Scale (VAS) alertness, and a larger increase from baseline in body sway and in saccadic reaction time compared with brivaracetam alone or ethanol alone. The immediate word recall scores were generally lower for brivaracetam when co-administered with ethanol.
Cardiac Electrophysiology
At a dose 4 times the maximum recommended dose, brivaracetam did not prolong the QT interval to a clinically relevant extent.
12.3 Pharmacokinetics
Brivaracetam tablets, oral solution, and injection can be used interchangeably. Brivaracetam exhibits linear and time-independent pharmacokinetics at the approved doses.
The pharmacokinetics of brivaracetam are similar when used as monotherapy or as adjunctive therapy for the treatment of partial-onset seizures.
Absorption
Brivaracetam is highly permeable and is rapidly and almost completely absorbed after oral administration. Pharmacokinetics is dose-proportional from 10 to 600 mg (a range that extends beyond the minimum and maximum single-administration dose levels described in Dosage and Administration [see Dosage and Administration (2.1)] ). The median Tmax for tablets taken without food is 1 hour (range 0.25 to 3 hours). Co-administration with a high-fat meal slowed absorption, but the extent of absorption remained unchanged. Specifically, when a 50 mg tablet was administered with a high-fat meal, Cmax (maximum brivaracetam plasma concentration during a dose interval, an exposure metric) was decreased by 37% and Tmax was delayed by 3 hours, but AUC (area under the brivaracetam plasma concentration versus time curve, an exposure metric) was essentially unchanged (decreased by 5%).
Distribution
Brivaracetam is weakly bound to plasma proteins (≤20%). The volume of distribution is 0.5 L/kg, a value close to that of the total body water. Brivaracetam is rapidly and evenly distributed in most tissues.Elimination
Metabolism
Brivaracetam is primarily metabolized by hydrolysis of the amide moiety to form the corresponding carboxylic acid metabolite, and secondarily by hydroxylation on the propyl side chain to form the hydroxy metabolite. The hydrolysis reaction is mediated by hepatic and extra-hepatic amidase. The hydroxylation pathway is mediated primarily by CYP2C19. In human subjects possessing genetic variations in CYP2C19, production of the hydroxy metabolite is decreased 2-fold or 10-fold, while the blood level of brivaracetam itself is increased by 22% or 42%, respectively, in individuals with one or both mutated alleles. CYP2C19 poor metabolizers and patients using inhibitors of CYP2C19 may require dose reduction. An additional hydroxy acid metabolite is created by hydrolysis of the amide moiety on the hydroxy metabolite or hydroxylation of the propyl side chain on the carboxylic acid metabolite (mainly by CYP2C9). None of the 3 metabolites are pharmacologically active.
Excretion
Brivaracetam is eliminated primarily by metabolism and by excretion in the urine. More than 95% of the dose, including metabolites, is excreted in the urine within 72 hours after intake. Fecal excretion accounts for less than 1% of the dose. Less than 10% of the dose is excreted unchanged in the urine. Thirty-four percent of the dose is excreted as the carboxylic acid metabolite in urine. The terminal plasma half-life (t1/2) is approximately 9 hours.Specific Populations
Age
Pediatric Patients (2 months to less than 16 years): An open-label, single-arm, multicenter, pharmacokinetic study with a 3-week evaluation period and fixed 3-step up-titration using brivaracetam oral solution was conducted in 99 pediatric patients 2 months to less than 16 years of age. In those patients, plasma concentrations were shown to be dose-proportional. The pediatric pharmacokinetic profile for brivaracetam was determined in a population pharmacokinetic analysis using sparse plasma concentration data obtained in three open-label studies in 255 adult and pediatric patients with epilepsy 2 months to 22 years of age that received intravenous, oral solution, or oral tablet formulations.
A weight-based dosing regimen is necessary to achieve brivaracetam exposures in pediatric patients 1 month to less than 16 years of age that are similar to those observed in adults treated at effective doses of brivaracetam [see Dosage and Administration (2.2)]. The estimated plasma clearance was 1.09 L/h, 1.81 L/h, and 3.11 L/h for pediatric patients weighing 11 kg, 20 kg, and 50 kg, respectively. In comparison, plasma clearance was estimated at 3.58 L/h in adult patients (70 kg body weight).
Geriatric Population: In a study in elderly subjects (65 to 79 years old; creatinine clearance 53 to 98 mL/min/1.73 m2) receiving brivaracetam 200 mg twice daily (2 times the highest recommended dosage), the plasma half-life of brivaracetam was 7.9 hours and 9.3 hours in the 65 to 75 and >75years groups, respectively. The steady-state plasma clearance of brivaracetam was slightly lower (0.76 mL/min/kg) than in young healthy controls (0.83 mL/min/kg).Sex
There were no differences observed in the pharmacokinetics of brivaracetam between male and female subjects.
Race/Ethnicity
A population pharmacokinetic analysis comparing Caucasian and non-Caucasian patients showed no significant pharmacokinetic difference.
Renal Impairment
A study in adult subjects with severe renal impairment (creatinine clearance <30 mL/min/1.73m2 and not requiring dialysis) revealed that the plasma AUC of brivaracetam was moderately increased(21%) relative to healthy controls, while the AUCs of the acid, hydroxy, and hydroxyacid metabolites were increased 3-fold, 4-fold, and 21-fold, respectively. The renal clearance of these inactive metabolites was decreased 10-fold. Brivaracetam has not been studied in patients undergoing hemodialysis [see Use in Specific Populations (8.6)].
Hepatic Impairment
A pharmacokinetic study in adult subjects with hepatic cirrhosis, Child-Pugh grades A, B, and C, showed 50%, 57%, and 59% increases in brivaracetam exposure, respectively, compared to matched healthy controls. The effect of hepatic impairment on brivaracetam pharmacokinetics in pediatric patients is expected to be comparable to the effect observed in adults [see Dosage and Administration (2.5) and Use in Specific Populations (8.7)].
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
Drug-Metabolizing Enzyme Inhibition
Brivaracetam did not inhibit CYP1A2, 2A6, 2B6, 2C8, 2C9, 2D6, or 3A4. Brivaracetam weakly inhibited CYP2C19 and would not be expected to cause significant inhibition of CYP2C19 in humans. Brivaracetam was an inhibitor of epoxide hydrolase, (IC50 = 8.2 μM), suggesting that brivaracetam can inhibit the enzyme in vivo.
Drug-Metabolizing Enzyme Induction
Brivaracetam at concentrations up to10 μM caused little or no change of mRNA expression of CYP1A2, 2B6, 2C9, 2C19, 3A4, and epoxide hydrolase. It is unlikely that brivaracetam will induce these enzymes in vivo.
Transporters
Brivaracetam was not a substrate of P-gp, MRP1, or MRP2. Brivaracetam did not inhibit or weakly inhibit BCRP, BSEP, MATE1, MATE2/K, MRP2, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, or P-gp, suggesting that brivaracetam is unlikely to inhibit these transporters in vivo.
In Vivo Assessment of Drug Interactions
Drug Interaction Studies with Antiepileptic Drugs (AEDs)
Potential interactions between brivaracetam (25 mg twice daily to 100 mg twice daily) and other AEDs were investigated in a pooled analysis of plasma drug concentrations from all Phase 2 and 3 studies and in a population exposure-response analysis of placebo-controlled, Phase 3 studies in adjunctive therapy in the treatment of partial-onset seizures. None of the interactions require changes in the dose of brivaracetam. Interactions with carbamazepine and phenytoin can be clinically important [see Drug Interactions (7.2) and (7.3)]. The interactions are summarized in Table 5.
Table 5: Drug Interactions Between Brivaracetam and Concomitant Antiepileptic DrugsConcomitant AED
Influence of AED on Brivaracetam
Influence of Brivaracetam on AED
Carbamazepine
26% decrease in plasma concentration
None forcarbamazepine
Increase of carbamazepine-epoxide metabolite*
[see Drug Interactions (7.2)]Lacosamide
No data
None
Lamotrigine
None
None
Levetiracetam
None
None
Oxcarbazepine
None
None on the active monohydroxy metabolite derivative (MHD)
Phenobarbital
19% decrease in plasma concentration
None
Phenytoin
21% decrease in plasma concentration
Up to 20% increase in plasma concentration
[see Drug Interactions (7.3)]**
Pregabalin
No data
None
Topiramate
None
None
Valproicacid
None
None
Zonisamide
No data
None
*Brivaracetam is a reversible inhibitor of epoxide hydrolase resulting in an increased concentration of carbamazepine epoxide, an active metabolite of carbamazepine. The carbamazepine epoxide plasma concentration increased up to 198% at a brivaracetam dose of 100 mg twice daily.
** At a supratherapeutic dose of 400 mg/day brivaracetam, there was a 20% increase in phenytoin plasma concentration.
Drug Interaction Studies with Other Drugs
Effect of Other Drugs on Brivaracetam
Co-administration with CYP inhibitors or transporter inhibitors is unlikely to significantly affect brivaracetam exposure.
Co-administration with rifampin decreases brivaracetam plasma concentrations by 45%, an effect that is probably the result of CYP2C19 induction [see Dosage and Administration (2.6) and Drug Interactions (7.1)].
Oral Contraceptives
Co-administration of brivaracetam 200 mg twice daily (twice the recommended maximum daily dosage) with an oral contraceptive containing ethinylestradiol (0.03 mg) and levonorgestrel (0.15 mg) reduced estrogen and progestin AUCs by 27% and 23%, respectively, without impact on suppression of ovulation. However, co-administration of brivaracetam 50 mg twice daily with an oral contraceptive containing ethinylestradiol (0.03 mg) and levonorgestrel (0.15 mg) did not significantly influence the pharmacokinetics of either substance. The interaction is not expected to be of clinical significance. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a carcinogenicity study in mice, oral administration of brivaracetam (0, 400, 550, or 700 mg/kg/day) for 104 weeks increased the incidence of liver tumors (hepatocellular adenoma and carcinoma) in male mice at the two highest doses tested. At the dose (400 mg/kg) not associated with an increase in liver tumors, plasma exposures (AUC) were approximately equal to those in humans at the maximum recommended dose (MRD) of 200 mg/day. Oral administration (0, 150, 230, 450, or 700 mg/kg/day) to rats for 104 weeks resulted in an increased incidence of thymus tumors (benign thymoma) in female rats at the highest dose tested. At the highest dose not associated with an increase in thymus tumors, plasma exposures were approximately 9 times those in humans at the MRD.
Mutagenesis
Brivaracetam was negative for genotoxicity in in vitro (Ames, mouse lymphoma, and CHO chromosomal aberration) and in vivo (rat bone marrow micronucleus) assays.
Impairment of Fertility
Oral administration of brivaracetam (0, 100, 200, or 400 mg/kg/day) to male and female rats prior to and throughout mating and early gestation produced no adverse effects on fertility. The highest dose tested was associated with plasma exposures approximately 6 (males) and 13 (females) times those in humans at the MRD.
-
14 CLINICAL STUDIES
The effectiveness of brivaracetam in partial-onset seizures with or without secondary generalization was established in 3 fixed-dose, randomized, double-blind, placebo-controlled, multicenter studies (Studies 1, 2, and 3), which included 1550 patients. Patients enrolled had partial-onset seizures that were not adequately controlled with 1 to 2 concomitant antiepileptic drugs (AEDs). In each of these studies, 72% to 86% of patients were taking 2 or more concomitant AEDs with or without vagal nerve stimulation. The median baseline seizure frequency across the 3 studies was 9 seizures per 28 days. Patients had a mean duration of epilepsy of approximately 23 years.
All trials had an 8-week baseline period, during which patients were required to have at least 8 partial-onset seizures. The baseline period was followed by a 12-week treatment period. There was no titration period in these studies. Study 1 compared doses of brivaracetam 50 mg/day and 100 mg/day with placebo. Study 2 compared a dose of brivaracetam 50 mg/day with placebo. Study 3 compared doses of brivaracetam 100 mg/day and 200 mg/day with placebo. Brivaracetam was administered in equally divided twice daily doses. Upon termination of brivaracetam treatment, patients were down-titrated over a 1-, 2-, and 4-week duration for patients receiving 25, 50, and 100 mg twice daily brivaracetam, respectively.
The primary efficacy outcome in Study 1 and Study 2 was the percent reduction in 7-day partial-onset seizure frequency over placebo, while the primary outcome for Study 3 was the percent reduction in 28-day partial-onset seizure frequency over placebo. The criteria for statistical significance for all 3 studies was p<0.05. Table 6 presents the primary efficacy outcome of the percent change in seizure frequency over placebo, based upon each study’s protocol-defined 7- and 28-day seizure frequency efficacy outcome.
Table 6: Percent Reduction in Partial-Onset Seizure Frequency over Placebo (Studies 1, 2, and 3)
Percent Reduction Over Placebo (%)
STUDY 1a
Placebo (n=100)
------
50 mg/day (n=99)
9.5
100 mg/day(n=100)
17.0
STUDY 2a
Placebo (n=96)
------
50 mg/day (n=101)
16.9*
STUDY 3b
Placebo (n=259)
-----
100mg/day (n=252)
25.2*
200mg/day (n= 249)
25.7*
* Statistically significant based on testing procedure with alpha = 0.05
a Based upon 7-day seizure frequency
b Based upon 28-day seizure frequency
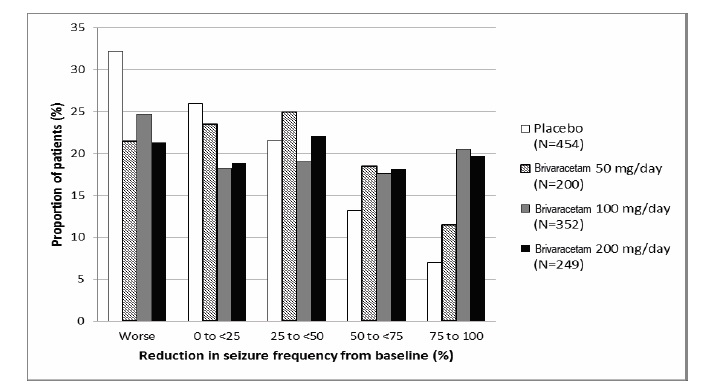
Figure 1 presents the percentage of patients by category of reduction from baseline in partial-onset seizure frequency per 28 days for all pooled patients in the 3 pivotal studies. Patients in whom the seizure frequency increased are shown at left as “worse.” Patients with an improvement in percent reduction from baseline partial-onset seizure frequency are shown in the 4 right-most categories.
Figure 1: Proportion of Patients by Category of Seizure Response for Brivaracetam and Placebo Across all Three Double-Blind Trials
Treatment with Levetiracetam
In Studies 1 and 2, which evaluated brivaracetam dosages of 50 mg and 100 mg daily, approximately 20% of the patients were on concomitant levetiracetam. Although the numbers of patients were limited, brivaracetam provided no added benefit when it was added to levetiracetam.
Although patients on concomitant levetiracetam were excluded from Study 3, which evaluated 100 and 200 mg daily, approximately 54% of patients in this study had prior exposure to levetiracetam. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Brivaracetam Tablets
- 10 mg are white to off white round, film-coated tablet debossed with “B10” on one side and “M” on other side, free from physical defects. They are supplied as follows:
Bottles of 60 tablets NDC: 72205-267-60
- 25 mg are pink, oval, film-coated tablet debossed with “B11” on one side and “M”on other side, free from physical defects. They are supplied as follows:
Bottles of 60 tablets NDC: 72205-268-60
Unit dose cartons of 100 tablets NDC: 72205-268-06- 50 mg are yellow, oval, film-coated tablet debossed with “B12” on one side and “M” on other side, free from physical defects..They are supplied as follows:
Bottles of 60 tablets NDC: 72205-269-60
Unit dose cartons of 100 tablets NDC: 72205-269-06- 75 mg are purple, oval, film-coated tablet debossed with “B13” on one side and “M” on other side, free from physical defects. They are supplied as follows:
Bottles of 60 tablets NDC: 72205-270-60
- 100 mg are orange, oval, film-coated tablet debossed with “B14” on one side and “M” on other side, free from physical defects. They are supplied as follows:
Bottles of 60 tablets NDC: 72205-271-60
Unit dose cartons of 100 tablets NDC: 72205-271-06 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). The Medication Guide accompanies the product and can also be accessed on www.novadozpharma.com or by calling 1-855-668-2369.
Suicidal Behavior and Ideation
Counsel patients, their caregivers, and/or families that antiepileptic drugs, including brivaracetam, may increase the risk of suicidal thoughts and behavior, and advise patients to be alert for the emergence or worsening of symptoms of depression; unusual changes in mood or behavior; or suicidal thoughts, behavior, or thoughts about self-harm. Advise patients, their caregivers, and/or families to report behaviors of concern immediately to a healthcare provider [see Warnings and Precautions (5.1)].
Neurological Adverse Reactions
Counsel patients that brivaracetam causes somnolence, fatigue, dizziness, and gait disturbance. These adverse reactions, if observed, are more likely to occur early in treatment but can occur at any time. Advise patients not to drive or operate machinery until they have gained sufficient experience on brivaracetam to gauge whether it adversely affects their ability to drive or operate machinery [see Warnings and Precautions (5.2)].
Psychiatric Adverse Reactions
Advise patients that brivaracetam causes changes in behavior (e.g., aggression, agitation, anger, anxiety, and irritability) and psychotic symptoms. Instruct patients to report these symptoms immediately to their healthcare provider [see Warnings and Precautions (5.3)].
Hypersensitivity: Bronchospasm and Angioedema
Advise patients that symptoms of hypersensitivity including bronchospasm and angioedema can occur with brivaracetam. Instruct them to seek immediate medical care should they experience signs and symptoms of hypersensitivity [see Warnings and Precautions (5.4)].
Serious Dermatologic Reactions
Advise patients of the early signs and symptoms of serious dermatologic adverse reactions and to report any occurrence immediately to a healthcare provider [seeWarnings and Precautions (5.5)].
Withdrawal of Antiepileptic Drugs
Advise patients not to discontinue use of brivaracetam without consulting with their healthcare provider. Brivaracetam should normally be gradually withdrawn to reduce the potential for increased seizure frequency and status epilepticus [see Warnings and Precautions (5.6)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during brivaracetam therapy. Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy[see Use in Specific Populations (8.1)].
Lactation
Counsel patients that brivaracetam, the active ingredient in brivaracetam tablets, is present in breast milk. Instruct patients to discuss with their healthcare provider if they are breastfeeding or intend to breastfeed [see Use in Specific Populations (8.2)].
Dosing Instructions
Counsel patients that brivaracetam may be taken with or without food. Instruct patients that brivaracetam tablets should be swallowed whole with liquid and not chewed or crushed[see Dosage and Administration (2.2)].
Manufactured by:
MSN Laboratories Private Limited
Telangana – 509 228,
INDIA
Distributed by:
Novadoz Pharmaceuticals LLC
Piscataway, NJ 08854 -3714
Issued : 11/2025
-
MEDICATION GUIDE
Brivaracetam
(briv" a ra' se tam)
Tablets, CVWhat is the most important information I should know about brivaracetam tablets?
Brivaracetam tablets is a federally controlled substance (CV) because it can be abused or lead to dependence. Keep brivaracetam tablets in a safe place to prevent misuse and abuse. Selling or giving away brivaracetam tablets may harm others and is against the law.
Like other antiepileptic drugs, brivaracetam tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500 people taking it.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, feeling angry, or being violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Do not stop brivaracetam tablets without first talking to a healthcare provider.
- Stopping brivaracetam tablets suddenly can cause serious problems.
- Stopping a seizure medicine suddenly can cause seizures that will not stop (status epilepticus).
What is brivaracetam tablets?
Brivaracetam tablet is a prescription medicine used to treat partial-onset seizures in people 1 month of age and older.
It is not known if brivaracetam is safe and effective in children younger than 1 month of age.
Who should not take brivaracetam tablets?
Do not take brivaracetam tablets if you are allergic to brivaracetam or any of the ingredients in brivaracetam tablets. See the end of this Medication Guide for a complete list of ingredients in brivaracetam tablets.What should I tell my healthcare provider before starting brivaracetam tablets?
Before taking brivaracetam tablets, tell your healthcare provider about all of your medical conditions, including if you:- have or had depression, mood problems, or suicidal thoughts or behavior.
- have liver problems.
- have abused or been dependent on prescription medicines, street drugs, or alcohol.
- are pregnant or plan to become pregnant. It is not known if brivaracetam tablets will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking brivaracetam tablets. You and your healthcare provider will have to decide if you should take brivaracetam tablets while you are pregnant. If you become pregnant while taking brivaracetam tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of brivaracetam tablets and other antiepileptic medicines during pregnancy.
- are breastfeeding or plan to breastfeed. Brivaracetam passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take brivaracetam tablets.
How should I take brivaracetam tablets?
- Take brivaracetam tablets exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how much brivaracetam tablets to take and when to take it.
- Your healthcare provider may change your dose if needed. Do not change your dose without talking to your healthcare provider.
- Take brivaracetam tablets with or without food.
- Swallow brivaracetam tablets whole with a liquid. Do not chew or crush brivaracetam tablets before swallowing.
- If you take too much brivaracetam tablets, call your Poison Control Center or go to the nearest emergency room right away.
What should I avoid while taking brivaracetam tablets?
Do not drive or operate machinery until you know how brivaracetam tablets affects you. Brivaracetam tablets may cause drowsiness, tiredness, dizziness, and problems with your balance and coordination.
What are the possible side effects of brivaracetam tablets?
Brivaracetam tablets may cause serious side effects, including:
- See “What is the most important information I should know about brivaracetam tablets?”
- Nervous system problems. Drowsiness, tiredness, and dizziness are common with brivaracetam, but can be severe. See “What should I avoid while taking brivaracetam tablets?” Brivaracetam tablets can also cause problems with balance and coordination.
- Mental (psychiatric) symptoms. Brivaracetam tablets can cause mood and behavior changes such as aggression, agitation, anger, anxiety, apathy, mood swings, depression, hostility, and irritability. Irritability and anxiety are common with brivaracetam, and can be severe. People who take brivaracetam tablets can also get psychotic symptoms such as hallucinations (seeing or hearing things that are really not there), delusions (false or strange thoughts or beliefs), and unusual behavior.
- Serious allergic reactions.Stop taking brivaracetam tablets and call your healthcare provider right away if you have any of these signs of a serious allergic reaction:
- swelling of your face, mouth, lips, gums, tongue, throat, or neck
- trouble breathing
- Serious skin rashes. Brivaracetam tablets may cause a severe rash with blisters and peeling skin. This may occur around the mouth, nose, eyes, and genitals or over much of the body. A mild rash may become a serious reaction and may cause death. Call your healthcare provider right away if you have a rash, skin blisters, sores in the mouth, or hives. Do not stop taking brivaracetam tablets without first talking to your healthcare provider.
- sleepiness
- feeling tired
- dizziness
- nausea and vomiting
These are not all the possible side effects of brivaracetam tablets. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider about any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store brivaracetam tablets?
- Store brivaracetam tablets at room temperature between 59°F to 86°F (15°C to 30°C).
General information about the safe and effective use of brivaracetam tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use brivaracetam for a condition for which it was not prescribed. Do not give brivaracetam tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about brivaracetam tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about brivaracetam tablets that is written for health professionals.What are the ingredients in brivaracetam tablets?
Active ingredient: brivaracetam
Tablet inactive ingredients: anhydrous lactose, croscarmellose sodium, lactose monohydrate, magnesium stearate, silicified microcrystalline cellulose. The tablet film-coating contains the inactive ingredients listed below:
- 10 mg tablets: titanium dioxide, polyethylene glycol, talc.
- 25 mg tablets: titanium dioxide, polyethylene glycol, talc, iron oxide red.
- 50 mg tablets: titanium dioxide, polyethylene glycol, talc, iron oxide yellow.
- 75 mg tablets: titanium dioxide, polyethylene glycol, talc, iron oxide red, black iron oxide.
- 100 mg tablets: titanium dioxide, polyethylene glycol, talc, FD&C yellow No.6
For more information, go to www.novadozpharma.com or call 1-855-668-2369.
Manufactured by:
MSN Laboratories Private Limited
Telangana – 509 228,
INDIA
Distributed by:
Novadoz Pharmaceuticals LLC
Piscataway, NJ 08854 -3714
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 11/2025
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Brivaracetam Tablets 10 mg-60s container label:
Brivaracetam Tablets 25 mg-60s container label:
Brivaracetam Tablets 25 mg-1s blister foil label:
Brivaracetam Tablets 25 mg-100s blister carton label:

Brivaracetam Tablets 50 mg-60s container label:
Brivaracetam Tablets 50 mg-1s blister foil label:
Brivaracetam Tablets 50 mg-100s blister carton label:
Brivaracetam Tablets 75 mg-60s container label:
Brivaracetam Tablets 100 mg-60s container label:
Brivaracetam Tablets 100 mg-1s blister foil label:
Brivaracetam Tablets 100 mg-100s blister carton label:

-
INGREDIENTS AND APPEARANCE
BRIVARACETAM
brivaracetam tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72205-267 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIVARACETAM (UNII: U863JGG2IA) (BRIVARACETAM - UNII:U863JGG2IA) BRIVARACETAM 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE Score no score Shape ROUND Size 5mm Flavor Imprint Code B10;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72205-267-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214921 11/13/2025 BRIVARACETAM
brivaracetam tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72205-268 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIVARACETAM (UNII: U863JGG2IA) (BRIVARACETAM - UNII:U863JGG2IA) BRIVARACETAM 25 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PINK Score no score Shape OVAL Size 9mm Flavor Imprint Code B11;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72205-268-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2026 2 NDC: 72205-268-06 100 in 1 CARTON 02/01/2026 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214921 11/13/2025 BRIVARACETAM
brivaracetam tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72205-269 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIVARACETAM (UNII: U863JGG2IA) (BRIVARACETAM - UNII:U863JGG2IA) BRIVARACETAM 50 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape OVAL Size 12mm Flavor Imprint Code B12;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72205-269-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2026 2 NDC: 72205-269-06 100 in 1 CARTON 02/01/2026 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214921 11/13/2025 BRIVARACETAM
brivaracetam tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72205-270 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIVARACETAM (UNII: U863JGG2IA) (BRIVARACETAM - UNII:U863JGG2IA) BRIVARACETAM 75 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color PURPLE Score no score Shape OVAL Size 13mm Flavor Imprint Code B13;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72205-270-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214921 11/13/2025 BRIVARACETAM
brivaracetam tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72205-271 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIVARACETAM (UNII: U863JGG2IA) (BRIVARACETAM - UNII:U863JGG2IA) BRIVARACETAM 100 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE Score no score Shape OVAL Size 15mm Flavor Imprint Code B14;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72205-271-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2026 2 NDC: 72205-271-06 100 in 1 CARTON 02/01/2026 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214921 11/13/2025 Labeler - Novadoz Pharmaceuticals LLC (081109687) Establishment Name Address ID/FEI Business Operations MSN LABORATORIES PRIVATE LIMITED 650786952 ANALYSIS(72205-267, 72205-268, 72205-269, 72205-270, 72205-271) , MANUFACTURE(72205-267, 72205-268, 72205-269, 72205-270, 72205-271)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.