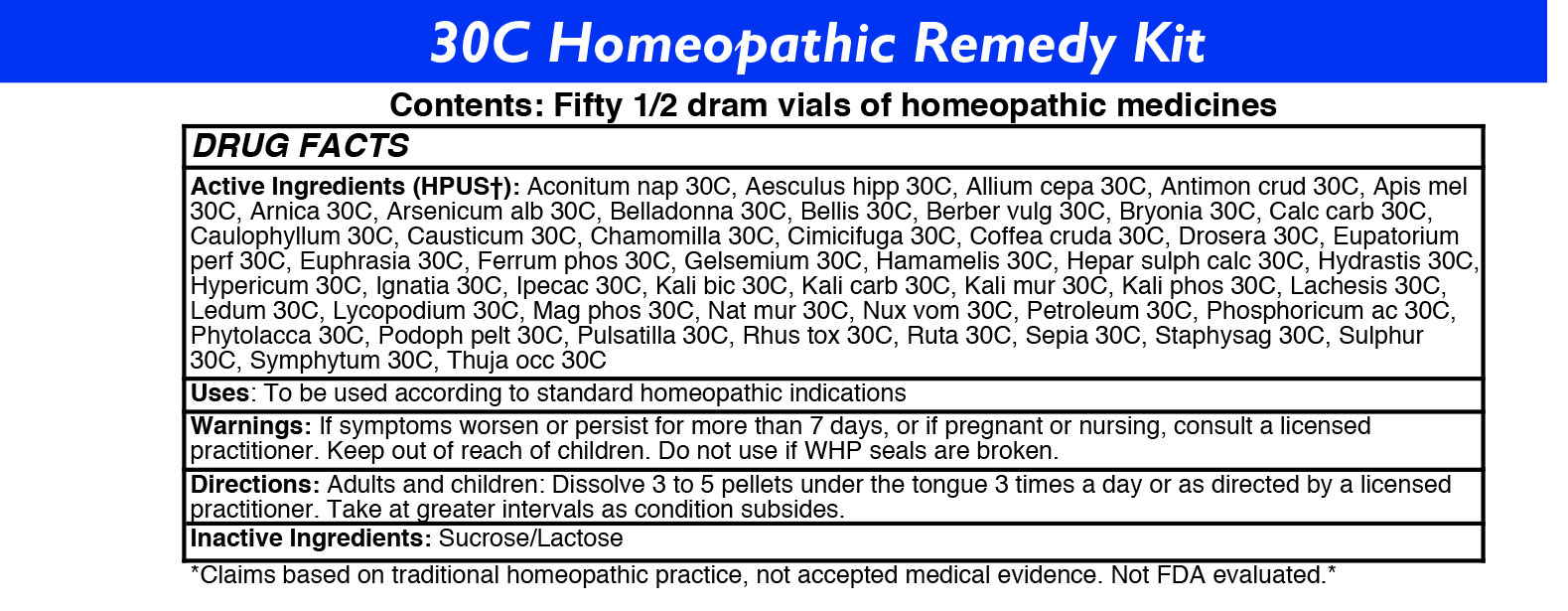
30C HOMEOPATHIC REMEDY KIT- 50 remedy kit kit
30C Homeopathic Remedy Kit by
Drug Labeling and Warnings
30C Homeopathic Remedy Kit by is a Homeopathic medication manufactured, distributed, or labeled by Washington Homeopathic Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENTS
Acon nap
Aesculus hipp
Allium cepa
Antimon crud
Apis mel
Arnica
Arsenicum alb
Belladonna
Bellis
Berber vulg
Bryonia
Calc carb
Caulophyllum
Causticum
Chamomilla
Cimicifuga
Coffea cruda
Drosera
Eupatorium perf
Euphrasia
Ferrum phos
Gelsemium
Hamamelis
Hepar sulph calc
Hydrastis
Hypericum
Ignatia
Ipecac
Kali bic
Kali carb
Kali mur
Kali phos
Lachesis
Ledum
Lycopodium
Mag phos
Nat mur
Nux vom
Petroleum
Phosphoricum ac
Phytolacca
Podoph pelt
Pulsatilla
Rhus tox
Ruta
Sepia
Staphysag
Sulphur
Symphytum
Thuja occ -
USES
To relieve symptoms of fear, hemorrhoids, head cold, warts, stings, bruises, vomiting, fever, bruised soreness, itching, worse motion, oberwork, cramps, hoarseness, irritability, minor back pain, sleeplessness, cough, flu-like symptoms, eye irritation, low fever, lethargy, varicose veins, croupiness, sinuses, shooting pain, sadness, nausea, sinuses, sour belching, congested ears, irritability, sore throat, black eyes, digestion, cramps, sneezing, vomiting, nausea, headache, sore throat, diarrhea, weeping, better motion, bruised feeling, indifference, anger, skin problems, prickling pain, and warts.
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS
Acon nap fear
Aesculus hipp hemorrhoids
Allium cepa head cold
Antimon crud warts
Apis mel stings
Arnica bruises
Arsenicum alb vomiting
Belladonna fever
Bellis bruised soreness
Berber vulg itching
Bryonia worse motion
Calc carb overwork
Caulophyllum cramps
Causticum hoarseness
Chamomilla irritability
Cimicifuga minor back pain
Coffea cruda sleeplessness
Drosera cough
Eupatorium perf flu-like symptoms
Euphrasia eye irritation
Ferrum phos low fever
Gelsemium lethargy
Hamamelis varicose veins
Hepar sulph calc croupiness
Hydrastis sinuses
Hypericum shooting pain
Ignatia sadness
Ipecac nausea
Kali bic sinuses
Kali carb sour belching
Kali mur congested ears
Kali phos irritability
Lachesis sore throat
Ledum black eyes
Lycopodium digestion
Mag phos cramps
Nat mur sneezing
Nux vom vomiting
Petroleum nausea
Phosphoricum ac headache
Phytolacca sore throat
Podoph pelt diarrhea
Pulsatilla weeping
Rhus tox better motion
Ruta bruised feeling
Sepia indifference
Staphysag anger
Sulphur skin problems
Symphytum prickling pain
Thuja occ warts
- STOP USE AND ASK DOCTOR
-
DIRECTIONS
Adults: Dissolve 3 to 5 under the tongue three times a day or as directed by a licensed practitioner. Take at greater intervals as condition subsides.
Children: Dissolve 3 to 5 under the tongue three times a day or as directed by a licensed practitioner. Take at greater intervals as condition subsides.
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
30C HOMEOPATHIC REMEDY KIT
50 remedy kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71919-821 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71919-821-18 50 in 1 KIT 02/21/2024 1 NDC: 71919-821-01 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 750 Part 2 1 VIAL, GLASS 750 Part 3 1 VIAL, GLASS 750 Part 4 1 VIAL, GLASS 750 Part 5 1 VIAL, GLASS 750 Part 6 1 VIAL, GLASS 750 Part 7 1 VIAL, GLASS 750 Part 8 1 VIAL, GLASS 750 Part 9 1 VIAL, GLASS 750 Part 10 1 VIAL, GLASS 750 Part 11 1 VIAL, GLASS 750 Part 12 1 VIAL, GLASS 750 Part 13 1 VIAL, GLASS 750 Part 14 1 VIAL, GLASS 750 Part 15 1 VIAL, GLASS 750 Part 16 1 VIAL, GLASS 750 Part 17 1 VIAL, GLASS 750 Part 18 1 VIAL, GLASS 750 Part 19 1 VIAL, GLASS 750 Part 20 1 VIAL, GLASS 750 Part 21 1 VIAL, GLASS 750 Part 22 1 VIAL, GLASS 750 Part 23 1 VIAL, GLASS 750 Part 24 1 VIAL, GLASS 750 Part 25 1 VIAL, GLASS 350 Part 26 1 VIAL, GLASS 750 Part 27 1 VIAL, GLASS 750 Part 28 1 VIAL, GLASS 750 Part 29 1 VIAL, GLASS 750 Part 30 1 VIAL, GLASS 750 Part 31 1 VIAL, GLASS 750 Part 32 1 VIAL, GLASS 750 Part 33 1 VIAL, GLASS 750 Part 34 1 VIAL, GLASS 750 Part 35 1 VIAL, GLASS 750 Part 36 1 VIAL, GLASS 750 Part 37 1 VIAL, GLASS 750 Part 38 1 VIAL, GLASS 750 Part 39 1 VIAL, GLASS 750 Part 40 1 VIAL, GLASS 750 Part 41 1 VIAL, GLASS 750 Part 42 1 VIAL, GLASS 750 Part 43 1 VIAL, GLASS 750 Part 44 1 VIAL, GLASS 750 Part 45 1 VIAL, GLASS 750 Part 46 1 VIAL, GLASS 750 Part 47 1 VIAL, GLASS 750 Part 48 1 VIAL, GLASS 750 Part 49 1 VIAL, GLASS 750 Part 50 1 VIAL, GLASS 750 Part 1 of 50 ACONITUM NAPELLUS KIT REFILL
aconitum napellus pelletProduct Information Item Code (Source) NDC: 68428-077 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-077-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 2 of 50 AESCULUS HIPP KIT REFILL
horse chestnut pelletProduct Information Item Code (Source) NDC: 68428-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-127-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/10/2011 Part 3 of 50 ALLIUM CEPA KIT REFILL
onion pelletProduct Information Item Code (Source) NDC: 68428-078 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-078-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 4 of 50 ANTIMONIUM CRUDUM KIT REFILL
antimony trisulfide pelletProduct Information Item Code (Source) NDC: 68428-131 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-131-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/10/2011 Part 5 of 50 APIS MELLIFICA KIT REFILL
apis mellifera pelletProduct Information Item Code (Source) NDC: 68428-080 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-080-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 6 of 50 ARNICA MONTANA KIT REFILL
arnica montana pelletProduct Information Item Code (Source) NDC: 68428-082 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-082-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 7 of 50 ARSENICUM ALBUM KIT REFILL
arsenic trioxide pelletProduct Information Item Code (Source) NDC: 68428-083 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-083-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 8 of 50 BELLADONNA KIT REFILL
atropa belladonna pelletProduct Information Item Code (Source) NDC: 68428-084 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-084-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 9 of 50 BELLIS PERENNIS KIT REFILL
bellis perennis pelletProduct Information Item Code (Source) NDC: 68428-133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-133-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/13/2011 Part 10 of 50 BERBERIS VULGARIS KIT REFILL
berberis vulgaris root bark pelletProduct Information Item Code (Source) NDC: 68428-134 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-134-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/13/2011 Part 11 of 50 BRYONIA ALBA KIT REFILL
bryonia alba root pelletProduct Information Item Code (Source) NDC: 68428-085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-085-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 12 of 50 CALCAREA CARBONICA KIT REFILL
oyster shell calcium carbonate, crude pelletProduct Information Item Code (Source) NDC: 68428-086 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-086-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 13 of 50 CAULOPHYLLUM THAL KIT REFILL
caulophyllum thalictroides root pelletProduct Information Item Code (Source) NDC: 68428-090 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-090-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 14 of 50 CAUSTICUM KIT REFILL
causticum pelletProduct Information Item Code (Source) NDC: 68428-091 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-091-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 15 of 50 CHAMOMILLA KIT REFILL
matricaria recutita pelletProduct Information Item Code (Source) NDC: 68428-092 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-092-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 16 of 50 CIMICIFUGA RACEMOSA KIT REFILL
black cohosh pelletProduct Information Item Code (Source) NDC: 68428-138 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-138-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/13/2011 Part 17 of 50 COFFEA CRUDA KIT REFILL
arabica coffee bean pelletProduct Information Item Code (Source) NDC: 68428-095 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-095-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 18 of 50 DROSERA ROTUNDIFOLIA KIT REFILL
drosera rotundifolia pelletProduct Information Item Code (Source) NDC: 68428-142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DROSERA ROTUNDIFOLIA (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-142-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/13/2011 Part 19 of 50 EUPATORIUM PERF KIT REFILL
eupatorium perfoliatum flowering top pelletProduct Information Item Code (Source) NDC: 68428-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-144-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 20 of 50 EUPHRASIA OFFICINALIS KIT REFILL
euphrasia stricta pelletProduct Information Item Code (Source) NDC: 68428-097 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-097-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 21 of 50 FERRUM PHOS KIT REFILL
ferrum phosphoricum pelletProduct Information Item Code (Source) NDC: 68428-098 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-098-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 22 of 50 GELSEMIUM SEMP KIT REFILL
gelsemium sempervirens root pelletProduct Information Item Code (Source) NDC: 68428-099 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-099-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 23 of 50 HAMAMELIS VIRGINIANA KIT REFILL
hamamelis virginiana root bark/stem bark pelletProduct Information Item Code (Source) NDC: 68428-146 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-146-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 24 of 50 HEPAR SULPH CALC KIT REFILL
calcium sulfide pelletProduct Information Item Code (Source) NDC: 68428-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-101-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 25 of 50 HYDRASTIS CANADENSIS
goldenseal pelletProduct Information Item Code (Source) NDC: 71919-768 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71919-768-01 350 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/25/2022 Part 26 of 50 HYPERICUM PERF KIT REFILL
hypericum perforatum pelletProduct Information Item Code (Source) NDC: 68428-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-102-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 27 of 50 IGNATIA AMARA KIT REFILL
strychnos ignatii seed pelletProduct Information Item Code (Source) NDC: 68428-103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-103-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 28 of 50 IPECACUANHA KIT REFILL
ipecac pelletProduct Information Item Code (Source) NDC: 68428-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-104-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 29 of 50 KALI BICHROMICUM KIT REFILL
potassium dichromate pelletProduct Information Item Code (Source) NDC: 68428-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-105-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 30 of 50 KALI CARBONICUM KIT REFILL
potassium carbonate pelletProduct Information Item Code (Source) NDC: 68428-147 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-147-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 31 of 50 KALI MURIATICUM KIT REFILL
potassium chloride pelletProduct Information Item Code (Source) NDC: 68428-148 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-148-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 32 of 50 KALI PHOSPHORICUM KIT REFILL
potassium phosphate, dibasic pelletProduct Information Item Code (Source) NDC: 68428-149 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-149-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 33 of 50 LACHESIS MUTUS KIT REFILL
lachesis muta venom pelletProduct Information Item Code (Source) NDC: 68428-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-106-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 34 of 50 LEDUM PALUSTRE KIT REFILL
ledum palustre twig pelletProduct Information Item Code (Source) NDC: 68428-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-107-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 35 of 50 LYCOPODIUM KIT REFILL
lycopodium clavatum spore pelletProduct Information Item Code (Source) NDC: 68428-108 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-108-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 36 of 50 MAGNESIA PHOS KIT REFILL
magnesium phosphate, dibasic trihydrate pelletProduct Information Item Code (Source) NDC: 68428-109 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-109-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 37 of 50 NATRUM MURIATICUM KIT REFILL
sodium chloride pelletProduct Information Item Code (Source) NDC: 68428-111 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-111-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 38 of 50 NUX VOMICA KIT REFILL
strychnos nux-vomica seed pelletProduct Information Item Code (Source) NDC: 68428-112 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-112-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 39 of 50 PETROLEUM KIT REFILL
kerosene pelletProduct Information Item Code (Source) NDC: 68428-154 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KEROSENE (UNII: 1C89KKC04E) (KEROSENE - UNII:1C89KKC04E) KEROSENE 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-154-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 40 of 50 PHOSPHORICUM ACIDUM KIT REFILL
phosphoric acid pelletProduct Information Item Code (Source) NDC: 68428-155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-155-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 41 of 50 PHYTOLACCA KIT REFILL
phytolacca americana root pelletProduct Information Item Code (Source) NDC: 68428-114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-114-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 42 of 50 PODOPHYLLUM PELT KIT REFILL
podophyllum pelletProduct Information Item Code (Source) NDC: 68428-156 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PODOPHYLLUM (UNII: 2S713A4VP3) (PODOPHYLLUM - UNII:2S713A4VP3) PODOPHYLLUM 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-156-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/20/2011 Part 43 of 50 PULSATILLA KIT REFILL
pulsatilla vulgaris pelletProduct Information Item Code (Source) NDC: 68428-115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-115-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 44 of 50 RHUS TOX KIT REFILL
toxicodendron radicans leaf pelletProduct Information Item Code (Source) NDC: 68428-117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOXICODENDRON RADICANS LEAF (UNII: CDH3461U7L) (TOXICODENDRON RADICANS LEAF - UNII:CDH3461U7L) TOXICODENDRON RADICANS LEAF 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-117-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 45 of 50 RUTA GRAVEOLENS KIT REFILL
ruta graveolens flowering top pelletProduct Information Item Code (Source) NDC: 68428-118 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-118-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 46 of 50 SEPIA KIT REFILL
sepia officinalis juice pelletProduct Information Item Code (Source) NDC: 68428-119 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-119-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 47 of 50 STAPHYSAGRIA KIT REFILL
delphinium staphisagria seed pelletProduct Information Item Code (Source) NDC: 68428-122 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-122-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 48 of 50 SULPHUR KIT REFILL
sulfur pelletProduct Information Item Code (Source) NDC: 68428-123 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-123-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 49 of 50 SYMPHYTUM OFF KIT REFILL
comfrey root pelletProduct Information Item Code (Source) NDC: 68428-124 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 30 [hp_C] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) SUCROSE (UNII: C151H8M554) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-124-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Part 50 of 50 THUJA OCCIDENTALIS KIT REFILL
thuja occidentalis leafy twig pelletProduct Information Item Code (Source) NDC: 68428-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68428-125-01 750 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/05/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/21/2024 Labeler - Washington Homeopathic Products (084929389) Establishment Name Address ID/FEI Business Operations Washington Homeopathic Products 084929389 manufacture(71919-821)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.