DICLOFENAC SODIUM solution
Diclofenac Sodium by
Drug Labeling and Warnings
Diclofenac Sodium by is a Prescription medication manufactured, distributed, or labeled by SOLA Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DICLOFENAC SODIUM TOPICAL SOLUTION safely and effectively. See full prescribing information for DICLOFENAC SODIUM TOPICAL SOLUTION.
DICLOFENAC SODIUM topical solution 1.5% w/w, for topical use
Initial U.S. Approval: 1988WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. ( 5.1)
- Diclofenac Sodium is contraindicated in the setting of coronary artery bypass graft (CABG) surgery. ( 4, 5.1)
- NSAIDs, cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and or GI bleeding are at greater risk for serious GI events. ( 5.2)
INDICATIONS AND USAGE
Diclofenac sodium topical solution is a nonsteroidal anti-inflammatory drug indicated for the treatment of signs and symptoms of osteoarthritis of the knee(s). ( 1)
DOSAGE AND ADMINISTRATION
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals.
- The recommended dose is 40 drops on each painful knee, 4 times a day. ( 2)
- Apply diclofenac sodium topical solution to clean, dry skin. ( 2.1)
- Dispense diclofenac sodium topical solution 10 drops at a time either directly onto the knee or first into the hand and then onto the knee. Spread diclofenac sodium topical solution evenly around front, back and sides of the knee. Repeat this procedure until 40 drops have been applied and the knee is completely covered with solution. ( 2.1)
- Wash hands completely after administering the product.
- Wait until the area is completely dry before covering with clothing or applying sunscreen, insect repellent, cosmetics, topical medications, or other substances.
- Until the treated knee(s) is completely dry, avoid skin-to-skin contact between other people and the treated knee(s). ( 2.2)
- Do not get diclofenac sodium topical solution in your eyes, nose or mouth.
DOSAGE FORMS AND STRENGTHS
Diclofenac sodium topical solution, USP 1.5% w/w ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hepatotoxicity:Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop. ( 5.3)
- Hypertension:Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure. ( 5.4, 7)
- Heart Failure and Edema:Avoid use of diclofenac sodium topical solution in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure. ( 5.5)
- Renal Toxicity:Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of diclofenac sodium topical solution in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal function. ( 5.6)
- Anaphylactic Reactions:Seek emergency help if an anaphylactic reaction occurs. ( 5.7)
- Exacerbation of Asthma Related to Aspirin Sensitivity:Diclofenac sodium topical solution is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity). ( 5.8)
- Serious Skin Reactions:Discontinue diclofenac sodium topical solution at first appearance of skin rash or other signs of hypersensitivity. ( 5.9)
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Discontinue and evaluate clinically. ( 5.10).
- Fetal Toxicity:Limit use of NSAIDs, including diclofenac sodium topical solution, between about 20 to 30 weeks in pregnancy due to the risk of oligohydramnios/fetal renal dysfunction. Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy due to the risks of oligohydramnios/fetal renal dysfunction and premature closure of the fetal ductus arteriosus. ( 5.11, 8.1).
- Hematologic Toxicity:Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia. ( 5.12, 7)
- Exposure to light:Avoid exposure of treated knee(s) to natural or artificial sunlight. ( 5.15)
- Eye Contact:Avoid contact of diclofenac sodium topical solution with eyes and mucosa. ( 5.16)
- Oral Nonsteroidal Anti-inflammatory Drugs:Avoid concurrent use with oral NSAIDs. ( 5.17)
ADVERSE REACTIONS
Most common adverse reactions with diclofenac sodium topical solution are application site reactions. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact SOLA Pharmaceuticals at 1-866-747-7365 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch
DRUG INTERACTIONS
- Drugs that Interfere with Hemostasis (e.g., warfarin, aspirin, SSRIs/SNRIs):Monitor patients for bleeding who are concomitantly using diclofenac sodium topical solution with drugs that interfere with hemostasis. Concomitant use of diclofenac sodium topical solution and analgesic doses of aspirin is not generally recommended. ( 7)
- ACE Inhibitors, Angiotensin Receptor Blockers (ARB), or Beta-Blockers:Concomitant use with diclofenac sodium topical solution may diminish the antihypertensive effect of these drugs. Monitor blood pressure. ( 7)
- ACE Inhibitors and ARBs:Concomitant use with diclofenac sodium topical solution in elderly, volume depleted, or those with renal impairment may result in deterioration of renal function. In such high risk patients, monitor for signs of worsening renal function. ( 7)
- Diuretics:NSAIDS can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects. ( 7)
- Digoxin:Concomitant use with diclofenac sodium topical solution can increase serum concentration and prolong half-life of digoxin. Monitor serum digoxin levels. ( 7)
USE IN SPECIFIC POPULATIONS
Use of NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs in pregnant women starting at 30 weeks gestation. ( 5.11, 8.1)
Infertility:NSAIDs are associated with reversible infertility. Consider withdrawal of diclofenac sodium topical solution in women who have difficulties conceiving. ( 8.3)See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Instructions
2.2 Special Precautions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
5.2 Gastrointestinal Bleeding, Ulceration and Perforation
5.3 Hepatotoxicity
5.4 Hypertension
5.5 Heart Failure and Edema
5.6 Renal Toxicity and Hyperkalemia
5.7 Anaphylactic Reactions
5.8 Exacerbation of Asthma Related to Aspirin Sensitivity
5.9 Serious Skin Reactions
5.10 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
5.11 Fetal Toxicity
5.12 Hematologic Toxicity
5.13 Masking of Inflammation and Fever
5.14 Laboratory Monitoring
5.15 Sun Exposure
5.16 Eye Exposure
5.17 Oral Nonsteroidal Anti-Inflammatory Drugs
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Studies in Osteoarthritis of the Knee
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of useand stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [see Warnings and Precautions (5.1)].
- Diclofenac sodium topical solution is contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4) and Warnings and Precautions (5.1)] .
Gastrointestinal Bleeding, Ulceration, and Perforation
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and or GI bleeding are at greater risk for serious GI events [see Warnings and Precautions (5.2)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Instructions
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warning and Precautions (5.2)].
For the relief of the signs and symptoms of osteoarthritis of the knee(s), the recommended dose is 40 drops per knee, 4 times a day.
Apply diclofenac sodium topical solution to clean, dry skin.
To avoid spillage, dispense diclofenac sodium topical solution 10 drops at a time either directly onto the knee or first into the hand and then onto the knee. Spread diclofenac sodium topical solution evenly around front, back and sides of the knee. Repeat this procedure until 40 drops have been applied and the knee is completely covered with solution.
To treat the other knee, if symptomatic, repeat the procedure
Application of diclofenac sodium topical solution in an amount exceeding or less than the recommended dose has not been studied and is therefore not recommended.
2.2 Special Precautions
- Avoid showering/bathing for at least 30 minutes after the application of diclofenac sodium topical solution to the treated knee.
- Wash and dry hands after use.
- Do not apply diclofenac sodium topical solution to open wounds.
- Avoid contact of diclofenac sodium topical solution with eyes and mucous membranes.
- Do not apply external heat and/or occlusive dressings to treated knees.
- Avoid wearing clothing over the diclofenac sodium topical solution-treated knee(s) until the treated knee is dry.
- Protect the treated knee(s) from natural or artificial sunlight.
- Wait until the treated area is dry before applying sunscreen, insect repellant, lotion, moisturizer, cosmetics, or other topical medication to the same knee you have just treated with diclofenac sodium topical solution.
- Until the treated knee(s) is completely dry, avoid skin-to-skin contact between other people and the treated knee(s).
- Do not use combination therapy with diclofenac sodium topical solution and an oral NSAID unless the benefit outweighs the risk and conduct periodic laboratory evaluations.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Diclofenac sodium topical solution is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to diclofenac or any components of the drug product [see Warnings and Precautions (5.7, 5.9)].
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.7, 5.8)].
- In the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as diclofenac, increases the risk of serious gastrointestinal (GI) events [see Warnings and Precautions (5.2)].
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke [see Contraindications (4)].Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post-MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.Avoid the use of diclofenac sodium topical solution in patients with a recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If diclofenac sodium topical solution is used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
5.2 Gastrointestinal Bleeding, Ulceration and Perforation
NSAIDs, including diclofenac, cause serious gastrointestinal (GI) adverse events including inflammation bleeding, ulceration, and perforation of the esophagus, stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occurred in approximately 1% of patients treated for 3 to 6 months, and in about 2% to 4% of patients treated for one year. However, even short-term NSAID therapy is not without risk.
Risk Factors for GI Bleeding, Ulceration, and Perforation
Patients with a prior history of peptic ulcer disease and/or GI bleeding who used NSAIDs had a greater than 10-fold increased risk for developing a GI bleed compared to patients without these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include longer duration of NSAID therapy; concomitant use of oral corticosteroids, aspirin, anticoagulants, or selective serotonin reuptake inhibitors (SSRIs); smoking; use of alcohol; older age; and poor general health status. Most postmarketing reports of fatal GI events occurred in elderly or debilitated patients. Additionally, patients with advanced liver disease and/or coagulopathy are at increased risk for GI bleeding.Strategies to Minimize the GI Risks in NSAID-treated patients:
- Use the lowest effective dosage for the shortest possible duration.
- Avoid administration of more than one NSAID at a time.
- Avoid use in patients at higher risk unless benefits are expected to outweigh the increased risk of bleeding. For such patients, as well as those with active GI bleeding, consider alternate therapies other than NSAIDs.
- Remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy.
- If a serious GI adverse event is suspected, promptly initiate evaluation and treatment, and discontinue diclofenac sodium topical solution until a serious GI adverse event is ruled out.
- In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, monitor patients more closely for evidence of GI bleeding [see Drug Interactions (7)].
5.3 Hepatotoxicity
In clinical trials, of oral diclofenac-containing products, meaningful elevations (i.e., more than 3 times the ULN) of AST (SGOT) were observed in about 2% of approximately 5,700 patients at some time during diclofenac treatment (ALT was not measured in all studies).
In a large, open-label, controlled trial of 3,700 patients treated with oral diclofenac for 2 to 6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of 3,700 patients and included marked elevations (greater than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3 to 8 times the ULN), and marked (greater than 8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis.
Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations.
In postmarketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
In a European retrospective population-based, case-controlled study, 10 cases of diclofenac associated drug-induced liver injury with current use compared with non-use of diclofenac were associated with a statistically significant 4-fold adjusted odds ratio of liver injury. In this particular study, based on an overall number of 10 cases of liver injury associated with diclofenac, the adjusted odds ratio increased further with female gender, doses of 150 mg or more, and duration of use for more than 90 days.
Physicians should measure transaminases at baseline and periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac.
If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), diclofenac sodium topical solution should be discontinued immediately.
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue diclofenac sodium topical solution immediately, and perform a clinical evaluation of the patient.
To minimize the potential risk for an adverse liver-related event in patients treated with diclofenac sodium topical solution, use the lowest effective dose for the shortest duration possible. Exercise caution when prescribing diclofenac sodium topical solution with concomitant drugs that are known to be potentially hepatotoxic (e.g., acetaminophen, antibiotics, antiepileptics).
5.4 Hypertension
NSAIDs, including diclofenac sodium topical solution, can lead to new onset of hypertension, or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking angiotensin converting enzyme (ACE) inhibitors, thiazide diuretics, or loop diuretics may have impaired response to these therapies when taking NSAIDs [see Drug Interactions (7)].
Monitor blood pressure (BP) closely during the initiation of NSAID treatment and throughout the course of therapy.
5.5 Heart Failure and Edema
The Coxib and traditional NSAID Trialists’ Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of diclofenac may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers [ARBs]) [see Drug Interactions (7)] .
Avoid the use of diclofenac sodium topical solution in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If diclofenac sodium topical solution is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
5.6 Renal Toxicity and Hyperkalemia
Renal Toxicity
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury.
Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, hypovolemia, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors or ARBs, and the elderly. Discontinuation of NSAID therapy was usually followed by recovery to the pretreatment state.
No information is available from controlled clinical studies regarding the use of diclofenac sodium topical solution in patients with advanced renal disease. The renal effects of diclofenac sodium topical solution may hasten the progression of renal dysfunction in patients with preexisting renal disease.
Correct volume status in dehydrated or hypovolemic patients prior to initiating diclofenac sodium topical solution. Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia during use of diclofenac sodium topical solution [see Drug Interactions (7)] . Avoid the use of diclofenac sodium topical solution in patients with advanced renal disease unless the benefits are expected to outweigh the risk of worsening renal function. If diclofenac sodium topical solution is used in patients with advanced renal disease, monitor patients for signs of worsening renal function.
5.7 Anaphylactic Reactions
Diclofenac has been associated with anaphylactic reactions in patients with and without known hypersensitivity to diclofenac and in patients with aspirin-sensitive asthma [see Contraindications (4)and Warnings and Precautions (5.8)].
Seek emergency help if an anaphylactic reaction occurs.
5.8 Exacerbation of Asthma Related to Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, diclofenac sodium topical solution is contraindicated in patients with this form of aspirin sensitivity. [see Contraindications (4)] . When diclofenac sodium topical solution is used in patients with preexisting asthma (without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
5.9 Serious Skin Reactions
NSAIDs, including diclofenac, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions, and to discontinue the use of diclofenac sodium topical solution at the first appearance of skin rash or any other sign of hypersensitivity. Diclofenac sodium topical solution is contraindicated in patients with previous serious skin reactions to NSAIDs [see Contraindications (4)] . Do not apply diclofenac sodium topical solution to open skin wounds, infections, inflammations, or exfoliative dermatitis, as it may affect absorption and tolerability of the drug.
Do not apply diclofenac sodium to open skin wounds, infections, inflammations, or exfoliative dermatitis, as it may affect absorption and tolerability of the drug.
5.10 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as diclofenac sodium topical solution. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue diclofenac sodium topical solution and evaluate the patient immediately.
5.11 Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs, including diclofenac sodium topical solution, in pregnant women at about 30 weeks gestation and later. NSAIDs, including diclofenac sodium topical solution, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.Oligohydramnios/Neonatal Renal Impairment:
Use of NSAIDs, including diclofenac sodium topical solution, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some post-marketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit diclofenac sodium topical solution use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if diclofenac sodium topical solution treatment extends beyond 48 hours. Discontinue diclofenac sodium topical solution if oligohydramnios occurs and follow up according to clinical practice [see Use in Specific Populations (8.1)].
5.12 Hematologic Toxicity
Anemia has occurred in NSAID-treated patients. This may be due to occult or gross blood loss, fluid retention, or an incompletely described effect on erythropoiesis. If a patient treated with diclofenac sodium topical solution has any signs or symptoms of anemia, monitor hemoglobin or hematocrit.
NSAIDs, including diclofenac sodium topical solution, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders, concomitant use of warfarin, other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase this risk. Monitor these patients for signs of bleeding [see Drug Interactions (7)] .
The effects of diclofenac sodium topical solution on platelet function were studied in 10 healthy subjects administered 80 drops four times a day for 7 days. There was no significant change in platelet aggregation following one week of treatment [see Clinical Pharmacology (12.4)].
5.13 Masking of Inflammation and Fever
The pharmacological activity of diclofenac sodium topical solution in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.
5.14 Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on long-term NSAID treatment with a CBC and a chemistry profile periodically [see Warnings and Precautions (5.2, 5.3, 5.6)].
5.15 Sun Exposure
Instruct patients to avoid exposure to natural or artificial sunlight on treated knee(s) because studies in animals indicated topical diclofenac treatment resulted in an earlier onset of ultraviolet light-induced skin tumors. The potential effects of diclofenac sodium topical solution on skin response to ultraviolet damage in humans are not known.
5.16 Eye Exposure
Avoid contact of diclofenac sodium topical solution with eyes and mucosa. Advise patients that if eye contact occurs, immediately wash out the eye with water or saline and consult a physician if irritation persists for more than an hour.
5.17 Oral Nonsteroidal Anti-Inflammatory Drugs
Concomitant use of oral NSAIDs with diclofenac sodium topical solution resulted in a higher rate of rectal hemorrhage, more frequent abnormal creatinine, urea and hemoglobin. Therefore, do not use combination therapy with diclofenac sodium topical solution and an oral NSAID unless the benefit outweighs the risk and conduct periodic laboratory evaluations.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events [see Warnings and Precautions (5.1)]
- GI Bleeding, Ulceration and Perforation [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
- Hypertension [see Warnings and Precautions (5.4)]
- Heart Failure and Edema [see Warnings and Precautions (5.5)]
- Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.6)]
- Anaphylactic Reactions [see Warnings and Precautions (5.7)]
- Serious Skin Reactions [see Warnings and Precautions (5.9)]
- Hematologic Toxicity [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to diclofenac sodium topical solution of 911 patients treated between 4 and 12 weeks (mean duration of 49 days) in seven Phase 3 controlled trials, as well as exposure of 793 patients treated in an open-label study, including 463 patients treated for at least 6 months, and 144 patients treated for at least 12 months. The population mean age was approximately 60 years, 89% of patients were Caucasians, 64% were females, and all patients had primary osteoarthritis. The most common adverse events with diclofenac sodium topical solution were application site skin reactions. These events were the most common reason for withdrawing from the studies.
Application Site Reactions
In controlled trials, the most common treatment-related adverse events in patients receiving diclofenac sodium topical solution were application site skin reactions. Application site reactions were characterized by one or more of the following: dryness, erythema, induration, vesicles, paresthesia, pruritus, vasodilation, acne, and urticaria. The most frequent of these reactions were dry skin (32%), contact dermatitis characterized by skin erythema and induration (9%), contact dermatitis with vesicles (2%) and pruritus (4%). In one controlled trial, a higher rate of contact dermatitis with vesicles (4%) was observed after treatment of 152 subjects with the combination of diclofenac sodium topical solution and oral diclofenac. In the open label uncontrolled long-term safety study, contact dermatitis occurred in 13% and contact dermatitis with vesicles in 10% of patients, generally within the first 6 months of exposure, leading to a withdrawal rate for an application site event of 14%.
Adverse Events Common to the NSAID Class
In controlled trials, subjects treated with diclofenac sodium topical solution experienced some adverse events associated with the NSAID class more frequently than subjects using placebo (constipation, diarrhea, dyspepsia, nausea, flatulence, abdominal pain, edema; see Table 1). The combination of diclofenac sodium topical solution and oral diclofenac, compared to oral diclofenac alone, resulted in a higher rate of rectal hemorrhage (3% vs. less than 1%), and more frequent abnormal creatinine (12% vs. 7%), urea (20% vs. 12%), and hemoglobin (13% vs. 9%), but no difference in elevation of liver transaminases.
Table 1lists all adverse reactions occurring in ≥ 1% of patients receiving diclofenac sodium topical solution, where the rate in the diclofenac sodium topical solution group exceeded placebo, from seven controlled studies conducted in patients with osteoarthritis. Since these trials were of different durations, these percentages do not capture cumulative rates of occurrence.
Table 1: Adverse Reactions occurring in ≥ 1% of patients treated with diclofenac sodium topical solution in placebo and oral diclofenac-controlled trials. Treatment Group: Diclofenac Sodium
Topical Solution
N=911Topical Placebo
N=332Adverse Reaction * N (%) N (%) - * Preferred Term according to COSTART
Dry Skin (Application Site) 292 (32) 17 (5) Contact Dermatitis (Application Site) 83 (9) 6 (2) Dyspepsia 72 (8) 13 (4) Abdominal Pain 54 (6) 10 (3) Flatulence 35 (4) 1 (<1) Pruritus (Application Site) 34 (4) 7 (2) Diarrhea 33 (4) 7 (2) Nausea 33 (4) 3 (1) Pharyngitis 40 (4) 13 (4) Constipation 29 (3) 1 (<1) Edema 26 (3) 0 Rash (Non-Application Site) 25 (3) 5 (2) Infection 25 (3) 8 (2) Ecchymosis 19 (2) 1 (<1) Dry Skin (Non-Application Site) 19 (2) 1 (<1) Contact Dermatitis, vesicles (Application Site) 18 (2) 0 Paresthesia (Non-Application Site) 14 (2) 3 (<1) Accidental Injury 22 (2) 7 (2) Pruritis (Non-Application Site) 15 (2) 2 (<1) Sinusitis 10 (1) 2 (<1) Halitosis 11 (1) 1 (<1) Application Site Reaction (not otherwise specified) 11 (1) 3 (<1) 6.2 Postmarketing Experience
In non-U.S. postmarketing surveillance, the following adverse reactions have been reported during post-approval use of diclofenac sodium topical solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole:abdominal pain, accidental injury, allergic reaction, asthenia, back pain, body odor, chest pain, edema, face edema, halitosis, headache, lack of drug effect, neck rigidity, pain
Cardiovascular:palpitation, cardiovascular disorder
Digestive:diarrhea, dry mouth, dyspepsia, gastroenteritis, decreased appetite, mouth ulceration, nausea, rectal hemorrhage, ulcerative stomatitis
Metabolic and Nutritional:creatinine increased
Musculoskeletal:leg cramps, myalgia
Nervous:depression, dizziness, drowsiness, lethargy, paresthesia, paresthesia at application site
Respiratory:asthma, dyspnea, laryngismus, laryngitis, pharyngitis
Skin and Appendages: At the Application Site:Adverse Reactions: contact dermatitis, contact dermatitis with vesicles, dry skin, pruritus, rash; Other Skin and Appendages:eczema, rash, pruritus, skin discoloration, urticaria
Special Senses:abnormal vision, blurred vision, cataract, ear pain, eye disorder, eye pain, taste perversion
-
7 DRUG INTERACTIONS
See Table 2for clinically significant drug interactions with diclofenac.
Table 2: Clinically Significant Drug Interactions with Diclofenac Drugs That Interfere with Hemostasis Clinical Impact: - Diclofenac and anticoagulants such as warfarin have a synergistic effect on bleeding. The concomitant use of diclofenac and anticoagulants have increased the risk of serious bleeding compared to the use of either drug alone.
- Serotonin release by platelets plays an important role in hemostasis. Case-control and cohort epidemiological studies showed that concomitant use of drugs that interfere with serotonin reuptake and an NSAID may potentiate the risk of bleeding more than an NSAID alone.
Intervention: Monitor patients with concomitant use of diclofenac sodium with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding [see Warnings and Precautions (5.12)] Aspirin Clinical Impact: Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone [see Warnings and Precautions (5.2)] Intervention: Concomitant use of diclofenac sodium and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding [see Warnings and Precautions (5.12)]. Diclofenac sodium is not a substitute for low dose aspirin for cardiovascular protection. ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-Blockers Clinical Impact: - NSAIDs may diminish the antihypertensive effect of angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or beta-blockers (including propranolol).
- In patients who are elderly, volume-depleted (including those on diuretic therapy), or have renal impairment, co-administration of an NSAID with ACE inhibitors or ARBs may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible.
Intervention: - During concomitant use of diclofenac sodium and ACE-inhibitors, ARBs, or beta-blockers, monitor blood pressure to ensure that the desired blood pressure is obtained.
- During concomitant use of diclofenac sodium and ACE-inhibitors or ARBs in patients who are elderly, volume-depleted, or have impaired renal function, monitor for signs of worsening renal function [see Warnings and Precautions (5.6)]
- When these drugs are administered concomitantly, patients should be adequately hydrated. Assess renal function at the beginning of the concomitant treatment and periodically thereafter.
Diuretics Clinical Impact: Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. Intervention: During concomitant use of diclofenac sodium with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects [see Warnings and Precautions (5.6)]. Digoxin Clinical Impact: The concomitant use of diclofenac with digoxin has been reported to increase the serum concentration and prolong the half-life of digoxin. Intervention: During concomitant use of diclofenac sodium and digoxin, monitor serum digoxin levels. Lithium Clinical Impact: NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. Intervention: During concomitant use of diclofenac sodium and lithium, monitor patients for signs of lithium toxicity. Methotrexate Clinical Impact: Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). Intervention: During concomitant use of diclofenac sodium and methotrexate, monitor patients for methotrexate toxicity. Cyclosporine Clinical Impact: Concomitant use of diclofenac sodium and cyclosporine may increase cyclosporine's nephrotoxicity. Intervention: During concomitant use of diclofenac sodium and cyclosporine, monitor patients for signs of worsening renal function. NSAIDs and Salicylates Clinical Impact: Concomitant use of diclofenac with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy [see Warnings and Precautions (5.2)]. Concomitant use of oral NSAIDs with diclofenac sodium has been evaluated in one Phase 3 controlled trial and in combination with oral diclofenac, compared to oral diclofenac alone, resulted in a higher rate of rectal hemorrhage (3% vs. less than 1%), and more frequent abnormal creatinine (12% vs. 7%), urea (20% vs. 12%) and hemoglobin (13% vs. 9%). Intervention: The concomitant use of diclofenac with other NSAIDs or salicylates is not recommended. Do not use combination therapy with diclofenac sodium and an oral NSAID unless the benefit outweighs the risk and conduct periodic laboratory evaluations. Pemetrexed Clinical Impact: Concomitant use of diclofenac sodium and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). Intervention: During concomitant use of diclofenac sodium and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 to 79 mL/min, monitor for myelosuppression, renal and GI toxicity.
NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed.
In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Use of NSAIDs, including diclofenac sodium, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of diclofenac sodium use between about 20 and 30 weeks of gestation, and avoid diclofenac sodium use at about 30 weeks of gestation and later in pregnancy (see Clinical Considerations, Data).Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including diclofenac sodium topical solution, at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus..Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.Data from observational studies regarding potential embryo-fetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. In animal reproduction studies, no evidence of teratogenicity was observed in mice, rats, or rabbits given diclofenac daily during the period of organogenesis at doses up to approximately 0.6, 0.6, and 1.3 times, respectively, the maximum recommended human dose (MRHD) of diclofenac sodium topical solution, despite the presence of maternal and fetal toxicity at these doses (see Data). Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as diclofenac, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including diclofenac sodium topical solution, can cause premature closure of the fetal ductus arteriosus (see Data).Oligohydramnios/Neonatal Renal Impairment
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If diclofenac sodium treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue diclofenac sodium and follow up according to clinical practice ( see Data).Data
Human DataPremature Closure of Fetal Ductus Arteriosus:
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment:
Published studies and postmarketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these postmarketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Animal Data
Reproductive and developmental studies in animals demonstrated that diclofenac sodium administration during organogenesis did not produce teratogenicity despite the induction of maternal toxicity and fetal toxicity in mice at oral doses up to 20 mg/kg/day (approximately 0.6 times the maximum recommended human dose [MRHD] of diclofenac sodium, 154 mg/day, based on body surface area (BSA) comparison), and in rats and rabbits at oral doses up to 10 mg/kg/day (approximately 0.6 and 1.3 times, respectively, the MRHD based on BSA comparison). Published reproductive and developmental studies of dimethyl sulfoxide (DMSO, the solvent used in diclofenac sodium) are equivocal as to potential teratogenicity.In rats, maternally toxic doses of diclofenac were associated with dystocia, prolonged gestation, reduced fetal weights and growth, and reduced fetal survival.
8.2 Lactation
Risk Summary
Based on available data, diclofenac may be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for diclofenac and any potential adverse effects on the breastfed infant from the diclofenac or from the underlying maternal condition.Data
One woman treated orally with a diclofenac salt, 150 mg/day, had a milk diclofenac level of 100 mcg/L, equivalent to an infant dose of about 0.03 mg/kg/day. Diclofenac was not detectable in breast milk in 12 women using diclofenac (after either 100 mg/day orally for 7 days or a single 50 mg intramuscular dose administered in the immediate postpartum period).8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs, including diclofenac sodium, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin-mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation. Consider withdrawal of NSAIDs, including diclofenac sodium, in women who have difficulties conceiving or who are undergoing investigation of infertility.8.5 Geriatric Use
Elderly patients, compared to younger patients, are a greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [see Warnings and Precautions (5.1, 5.2, 5.3, 5.6, 5.14)].
Of the 911 patients treated with diclofenac sodium topical solution in seven controlled, Phase 3 clinical trials, 444 subjects were 65 years of age and over. There was no age-related difference in the incidence of adverse events. Of the 793 patients treated with diclofenac sodium topical solution in one open-labeled safety trial, 334 subjects were 65 years of age and over including 107 subjects 75 and over. There was no difference in the incidence of adverse events with long-term exposure to diclofenac sodium topical solution for this elderly population.
-
10 OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [see Warnings and Precautions (5.1, 5.2. 5.4, 5.6)].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Emesis is not recommended due to a possibility of aspiration and subsequent respiratory irritation by DMSO contained in diclofenac sodium topical solution. Consider activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding. For additional information about overdose treatment, contact a poison control center (1-800-222-1222).
-
11 DESCRIPTION
Diclofenac sodium topical solution, USP 1.5% is a nonsteroidal anti-inflammatory drug, available as a clear, colorless to faintly pink-orange solution for topical application.
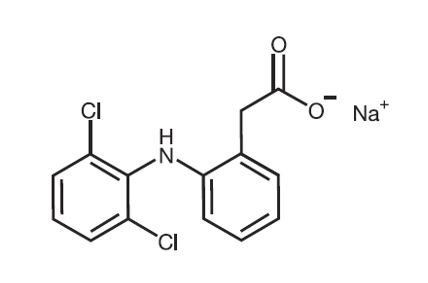
Diclofenac sodium topical solution, USP contains 1.5% w/w diclofenac sodium, USP, a benzeneacetic acid derivative that is a nonsteroidal anti-inflammatory drug (NSAID), designated chemically as 2-[(2,6-dichlorophenyl)amino]-benzeneacetic acid, monosodium salt. It is a white to off-white crystalline powder, hygroscopic, and odorless that is freely soluble in methanol, soluble in alcohol, and sparingly soluble in water. The molecular weight is 318.13. Its molecular formula is C 14H 10Cl 2NNaO 2and it has the following structural formula:
Each 1 mL of solution contains 16.05 mg of diclofenac sodium, USP. The inactive ingredients in diclofenac sodium topical solution, USP include: alcohol, dimethyl sulfoxide USP (DMSO, 45.5% w/w), glycerin, propylene glycol, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Diclofenac has analgesic, anti-inflammatory, and antipyretic properties.
The mechanism of action of diclofenac sodium topical solution, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Diclofenac is a potent inhibitor of prostaglandin synthesis in vitro. Diclofenac concentrations reached during therapy have produced in vivoeffects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because diclofenac is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.3 Pharmacokinetics
After topical administration to healthy human volunteers of single and multiple maximum doses of diclofenac sodium topical solution, 40 drops (approximately 1.2 mL) to each knee (80 drops total dose), the following diclofenac pharmacokinetic parameters were obtained: (see Table 3).
Table 3: Single-Dose (80 drops) and Multiple Dose (80 drops four times daily for 7 days) Diclofenac Sodium Topical Solution Pharmacokinetic Parameters Pharmacokinetic
ParametersDiclofenac sodium
Normal Adults [N=18]
(Age: 18-55 years)Normal Adults [N=19]
(Age: 18-55 years)
Single Dose Multiple Dose
Four times daily for 7 daysAUC 0-t 177.5 ± 72.6 ng.h/mL 695.4 ± 348.9 ng.h/mL
AUC 0-inf 196.3 ± 68.5 ng.h/mL 745.2 ± 374.7 ng.h/mL
Plasma C max 8.1 ± 5.9 ng/mL 19.4 ± 9.3 ng/mL
Plasma T max(h) 11.0 ± 6.4 4.0 ± 6.5
Plasma t 1/2(h) 36.7 ± 20.8 79.0 ± 38.1
K el(h -1) 0.024 ± 0.010 0.011 ± 0.004
CL/F (L/h) 244.7 ± 84.7 1 -
1Apparent total body clearance
Absorption
Diclofenac systemic exposure from diclofenac sodium topical solution application (4 times daily for 1 week) was approximately 1/3 of the diclofenac systemic exposure from the diclofenac topical gel application (twice daily for 4 weeks).Distribution
Diclofenac is more than 99% bound to human serum proteins, primarily to albumin.Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
Elimination
MetabolismFive diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4',5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. The major diclofenac metabolite, 4'-hydroxy-diclofenac, has very weak pharmacologic activity. The formation of 4'-hydroxy diclofenac is primarily mediated by CPY2C9. Both diclofenac and its oxidative metabolites undergo glucuronidation or sulfation followed by biliary excretion. Acylglucuronidation mediated by UGT2B7 and oxidation mediated by CPY2C8 may also play a role in diclofenac metabolism. CYP3A4 is responsible for the formation of minor metabolites, 5-hydroxy and 3'-hydroxy-diclofenac.
Special Populations
Drug Interaction Studies
Aspirin:When NSAIDs were administered with aspirin, the protein binding of NSAIDs were reduced, although the clearance of free NSAID was not altered. The clinical significance of this interaction is not known. See Table 1for clinically significant drug interactions of NSAIDs with aspirin [see Drug Interactions (7)]. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies in mice and rats administered diclofenac sodium as a dietary constituent for 2 years resulted in no significant increases in tumor incidence at doses up to 2 mg/kg/day corresponding to approximately 0.35- and 0.7-fold (mouse and rat, respectively) of the maximum recommended human topical dose (MRHD) of diclofenac sodium topical solution (based on apparent bioavailability and body surface area comparison).In a dermal carcinogenicity study conducted in albino mice, daily topical applications of diclofenac sodium for two years at concentrations up to 0.035% diclofenac sodium (a 43-fold lower diclofenac sodium concentration than present in diclofenac sodium topical solution) did not increase neoplasm incidence.
In a photococarcinogenicity study conducted in hairless mice, topical application of diclofenac sodium at doses up to 0.035% diclofenac sodium (a 43-fold lower diclofenac sodium concentration than present in diclofenac sodium topical solution) resulted in an earlier median time of onset of tumors.
Mutagenesis
Diclofenac was not mutagenic or clastogenic in a battery of genotoxicity tests that included the bacterial reverse mutation assay, in vitromouse lymphoma point mutation assay, chromosomal aberration studies in Chinese hamster ovarian cells in vitro, and in vivorat chromosomal aberration assay of bone marrow cells.Impairment of Fertility
Fertility studies have not been conducted with diclofenac sodium topical solution. Diclofenac sodium administered to male and female rats at doses up to 4 mg/kg/day (1.4-fold of the MRHD of diclofenac sodium topical solution based on apparent bioavailability and body surface area comparison) did not affect fertility. Studies have not been conducted to determine the safety of DMSO on fertility.13.2 Animal Toxicology and/or Pharmacology
Ocular Effects
No adverse effects were observed using indirect ophthalmoscopy after multiple-daily dermal application to rats for 26 weeks and minipigs for 52 weeks of DMSO at twice the concentration found in diclofenac sodium topical solution. Published studies of dermal or oral administration of DMSO to rabbits, dogs and pigs described refractive changes of lens curvature and cortical fibers indicative of myopic changes and/or incidences of lens opacity or discoloration when evaluated using slit-lamp biomicroscopy examination, although no ocular abnormalities were observed in rhesus monkeys during daily oral or dermal treatment with DMSO for 9 to 18 months.
-
14 CLINICAL STUDIES
14.1 Studies in Osteoarthritis of the Knee
The use of diclofenac sodium topical solution for the treatment of the signs and symptoms of osteoarthritis of the knee was evaluated in two double-blind controlled trials conducted in the U.S. and Canada, involving patients treated with diclofenac sodium topical solution at a dose of 40 drops four times a day for 12 weeks. Diclofenac sodium topical solution was compared to topical placebo (2.3% DMSO with other excipients) and/or topical vehicle solution (45.5% w/w DMSO with other excipients), applied directly to the study knee. In both trials, diclofenac sodium topical solution treatment resulted in statistically significant clinical improvement compared to placebo and/or vehicle, in all three primary efficacy variables–pain, physical function (Western Ontario and McMaster Universities LK3.1 OA Index (WOMAC) pain and physical function dimensions) and Patient Overall Health Assessment (POHA)/Patient Global Assessment (PGA). Numerical results are summarized in Tables 4and 5.
Table 4: Change in treatment outcomes after 12 weeks of treatment in one study of efficacy of diclofenac sodium topical solution Efficacy Variable Study I
Mean baseline score and mean change in efficacy variables after 12 weeks of treatmentMean Baseline score Diclofenac Sodium Topical Solution
N=154Topical placebo *
N=155Topical vehicle †
N=161- * placebo formulation included 2.3% DMSO
- † vehicle formulation included 45.5% DMSO
WOMAC pain score (Likert 3.1, 0-20) 13 -6.0 -4.7 -4.7 WOMAC physical function (Likert 3.1, 0-68) 42 -15.7 -12.3 -12.1 POHA (0-4) 2.3 -1.0 -0.4 -0.6 Table 5: Change in treatment outcomes after 12 weeks of treatment in one study of efficacy of diclofenac sodium topical solution Efficacy Variable Study II
Mean baseline score and mean change in efficacy variables after 12 weeks of treatmentMean Baseline score Diclofenac Sodium Topical Solution
N=164Topical vehicle *
N=162- * vehicle formulation included 45.5% DMSO
WOMAC pain score (Likert 3.1, 0-20) 13 -5.9 -4.4 WOMAC physical function (Likert 3.1, 0-68) 42 -15.3 -10.3 POHA (0-4) 3.1 -1.3 -1.0 -
16 HOW SUPPLIED/STORAGE AND HANDLING
Diclofenac sodium topical solution, USP 1.5%w/w is supplied as a clear, colorless to faintly pink-orange solution containing 16.05 mg of diclofenac sodium, USP per 1 mL of solution, in a white high density polyethylene bottle with a white low-density dropper cap. It is available as follows:
150 mL bottle NDC 70512-810-05
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) and Instructions for Use that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with diclofenac sodium topical solution and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [see Warnings and Precautions (5.1)].Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [see Warnings and Precautions (5.2)].Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, instruct patients to stop diclofenac sodium and seek immediate medical therapy [see Warnings and Precautions (5.3)].Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [see Warnings and Precautions (5.5)].Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur [see Contraindications (4)and Warnings and Precautions (5.7)].Serious Skin Reactions, including DRESS
Advise patients to stop taking diclofenac sodium immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible [see Warnings and Precautions(5.9, 5.10)] .Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including diclofenac sodium topical solution, may be associated with a reversible delay in ovulation [see Use in Specific Populations (8.3)].Fetal Toxicity
Inform pregnant women to avoid use of diclofenac sodium and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with diclofenac sodium is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [see Warnings and Precautions (5.11)and Use in Specific Populations (8.1)].Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of diclofenac sodium with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy [see Warnings and Precautions (5.2)and Drug Interactions (7)]. Alert patients that NSAIDs may be present in "over-the-counter" medications for treatment of colds, fever, or insomnia.Use of NSAIDs and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with diclofenac sodium until they talk to their healthcare provider [see Drug Interactions (7)].Eye Exposure
Instruct patients to avoid contact of diclofenac sodium with the eyes and mucosa. Advise patients that if eye contact occurs, immediately wash out the eye with water or saline and consult a physician if irritation persists for more than an hour.Prevention of Secondary Exposure
Instruct patients to avoid skin-to-skin contact between other people and the knee(s) to which diclofenac sodium was applied until the knee(s) is completely dry.Application Site Reactions
Diclofenac sodium can cause a localized skin reaction at the application site. Advise patients to contact their physicians as soon as possible if they develop any type of localized application site rash.Special Application Instructions
- Instruct patients not to apply diclofenac sodium to open skin wounds, infections, inflammations, or exfoliative dermatitis, as it may affect absorption and reduce tolerability of the drug.
- Instruct patients to wait until the area treated with diclofenac sodium topical solution is completely dry before applying sunscreen, insect repellant, lotion, moisturizer, cosmetics, or other topical medication.
- Instruct patients to minimize or avoid exposure of treated knee(s) to natural or artificial sunlight.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
SOLA Pharmaceuticals
Baton Rouge, LA 70810
Rev. 04-2023-00Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs) This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised 04-2023-00What is the most important information I should know about medicines called Nonsteroidal Anti- inflammatory Drugs (NSAIDs)? NSAIDs can cause serious side effects, including: -
Increased risk of a heart attack or stroke that can lead to death.This risk may happen early in treatment and may increase:
- with increasing doses of NSAIDs
- with longer use of NSAIDs
Do not take NSAIDs right before or after a heart surgery called a “coronary artery bypass graft (CABG)."
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
-
Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
- anytime during use
- without warning symptoms
- that may cause death
The risk of getting an ulcer or bleeding increases with:
- past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
- taking medicines called “corticosteroids”, “anticoagulants”, “SSRIs”, or “SNRIs”
- increasing doses of NSAIDs
- longer use of NSAIDs
- smoking
- drinking alcohol
- older age
- poor health
- advanced liver disease
- bleeding problems
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain.Who should not take NSAIDs?
Do not take NSAIDs:
- if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs.
- right before or after heart bypass surgery.
Before taking NSAIDS, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems
- have high blood pressure
- have asthma
- are pregnant or plan to become pregnant. Taking NSAIDs at about 20 weeks of pregnancy or later may harm your unborn baby. If you need to take NSAIDs for more than 2 days when you are between 20 and 30 weeks of pregnancy, your healthcare provider may need to monitor the amount of fluid in your womb around your baby.
You should not take NSAIDs after about 30 weeks of pregnancy.
- are breastfeeding or plan to breast feed.
What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
See “What is the most important information I should know about medicines called Nonsteroidal Anti- inflammatory Drugs (NSAIDs)?”
- new or worse high blood pressure
- heart failure
- liver problems including liver failure
- kidney problems including kidney failure
- low red blood cells (anemia)
- life-threatening skin reactions
-
Other side effects of NSAIDs include:stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness.
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
- nausea
- more tired or weaker than usual
- diarrhea
- itching
- your skin or eyes look yellow
- indigestion or stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms, legs, hands and feet
These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
- Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
Manufactured for:
SOLA Pharmaceuticals
Baton Rouge, LA 70810
For more information, go to www.amneal.com or call 1-877-835-5472
-
Increased risk of a heart attack or stroke that can lead to death.This risk may happen early in treatment and may increase:
- MEDICATION GUIDE
-
Instructions for UseDiclofenac (dye-KLOE-fen-ak) Sodium Topical Solution, USP 1.5%
Read the Medication Guide that comes with diclofenac sodium topical solution first. Be sure that you read, understand and follow these Instructions for Use before you use diclofenac sodium topical solution for the first time.
Important: For use on the skin only (topical). Do not get diclofenac sodium topical solution in your eyes, nose or mouth.
Before you use diclofenac sodium topical solution:
- Apply diclofenac sodium topical solution exactly as your healthcare provider tells you. Talk with your healthcare provider or pharmacist if you are not sure.
- Only use diclofenac sodium topical solution to treat pain from osteoarthritis in your knee or knees.
- Apply diclofenac sodium topical solution on clean, dry skin that does not have any cuts, infections or rashes.
- Use diclofenac sodium topical solution 4 times each day on your knee or knees as prescribed.
- Your total dose for each knee is 40 drops of diclofenac sodium topical solution, each time you use it.
- If you get diclofenac sodium topical solution in your eyes, rinse your eyes right away with water or saline. Call your healthcare provider if your eyes are irritated for more than one hour.
Steps for using diclofenac sodium topical solution:
Step 1. Wash your hands with soap and water before applying diclofenac sodium topical solution.
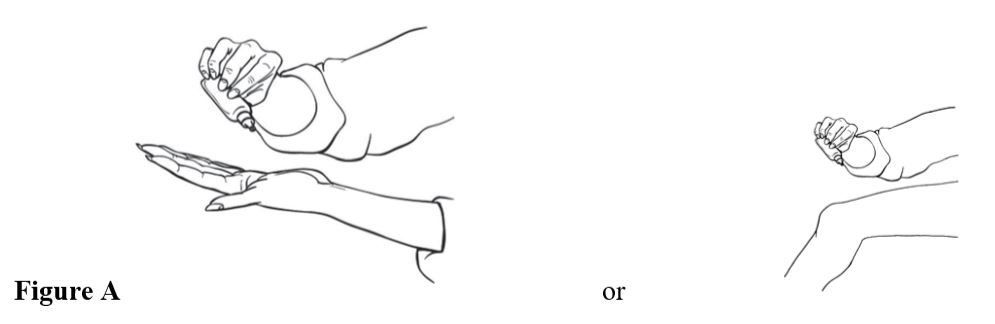
Step 2. Put 10 drops of diclofenac sodium topical solution eitheron your hand ordirectly on your knee (see Figure A).
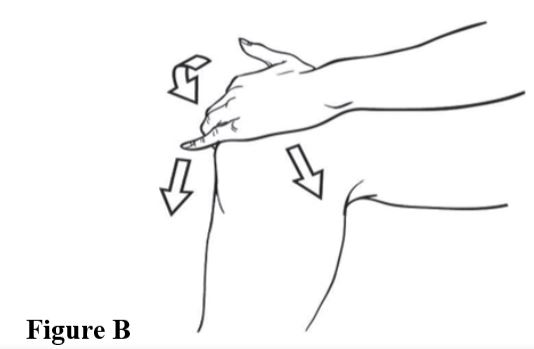
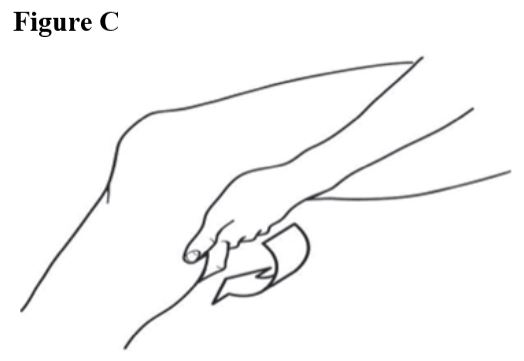
Step 3. Spread diclofenac sodium topical solution evenly on the front, back and sides of your knee (see Figures Band C). Repeat steps 2 and 3, three times so that your knee is completely covered with a totalof 40 drops of diclofenac sodium topical solution.
Step 4. If your healthcare provider has prescribed diclofenac sodium topical solution for both knees, repeat steps 2 and 3 for the other knee.
After you use diclofenac sodium topical solution:
- Wash your hands with soap and water right away after applying diclofenac sodium topical solution.
Do not:
- touch the treated knee or allow another person to touch the knee treated with diclofenac sodium topical solution until your knee is completely dry.
- cover your knee with clothing until your knee is completely dry.
- put sunscreen, insect repellant, lotion, moisturizer, cosmetics, or other topical medicines on your knee until it is completely dry.
- take a shower or a bath for at least 30 minutes after you put diclofenac sodium topical solution on your knee.
- use heating pads or cover the treated area with bandages where you have applied diclofenac sodium topical solution.
- use sunlamps and tanning beds. Protect your treated knee from sunlight. Wear clothes that cover your skin if you have to be in sunlight.
How should I store diclofenac sodium topical solution?
- Store diclofenac sodium topical solution at room temperature between 68°F to 77°F (20°C to 25°C).
Keep diclofenac sodium topical solution and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
*Trademarks are the property of their respective owners.
Manufactured for:
SOLA Pharmaceuticals
Baton Rouge, LA 70810
Rev. 04-2023-00 -
PRINCIPAL DISPLAY PANEL
NDC 70512- 810-05
Diclofenac
Sodium
Topical Solution USP, 1.5% w/w
NET CONTENTS 5 fl oz (150 mL)
Avoid contact with the eyes or mucous membranes.
FOR EXTERNAL USE ONLY
Usual dosage:
40 drops to a knee, four times a day.
Dispense Enclosed Medication GuideTo Each Patient.
Each mL contains Active Ingredient:
diclofenac sodium, USP, 16.05 mg
SOLA
PHARMACEUTICALS
Rx only
Rev. 04-2023-00
LOT
EXP
Manufactured for:
SOLA Pharmaceuticals
Baton Rouge, LA. 70810
NDC 70512- 810-05
Diclofenac
Sodium
Topical Solution USP, 1.5% w/w
NET CONTENTS 5 fl oz (150 mL)
Dispense Enclosed Medication
Guide To Each Patient.
Avoid contact with the eyes or
mucous membranes.
FOR EXTERNAL USE ONLY
SOLA
PHARMACEUTICALS
Rx only
Rev. 04-2023-00
Manufactured for:
SOLA Pharmaceuticals
Baton Rouge, LA. 70810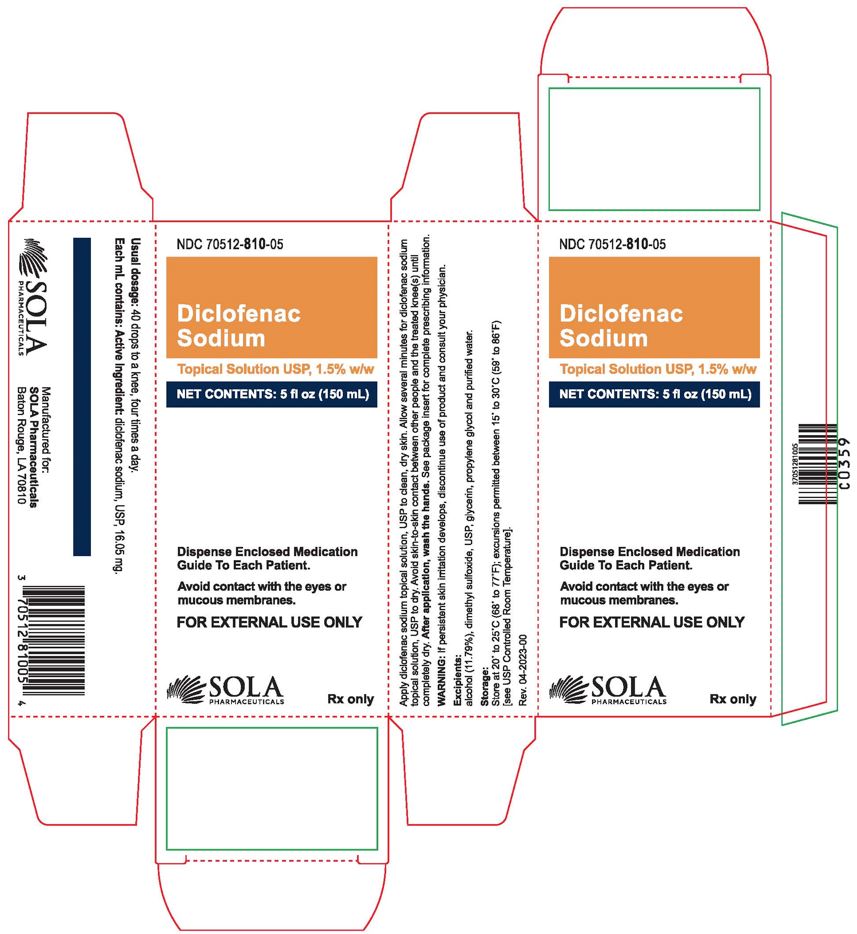
-
INGREDIENTS AND APPEARANCE
DICLOFENAC SODIUM
diclofenac sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70512-810 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICLOFENAC SODIUM (UNII: QTG126297Q) (DICLOFENAC - UNII:144O8QL0L1) DICLOFENAC SODIUM 16.05 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70512-810-05 1 in 1 BOX 07/01/2023 1 150 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206116 07/01/2023 Labeler - SOLA Pharmaceuticals, LLC (080121345)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.