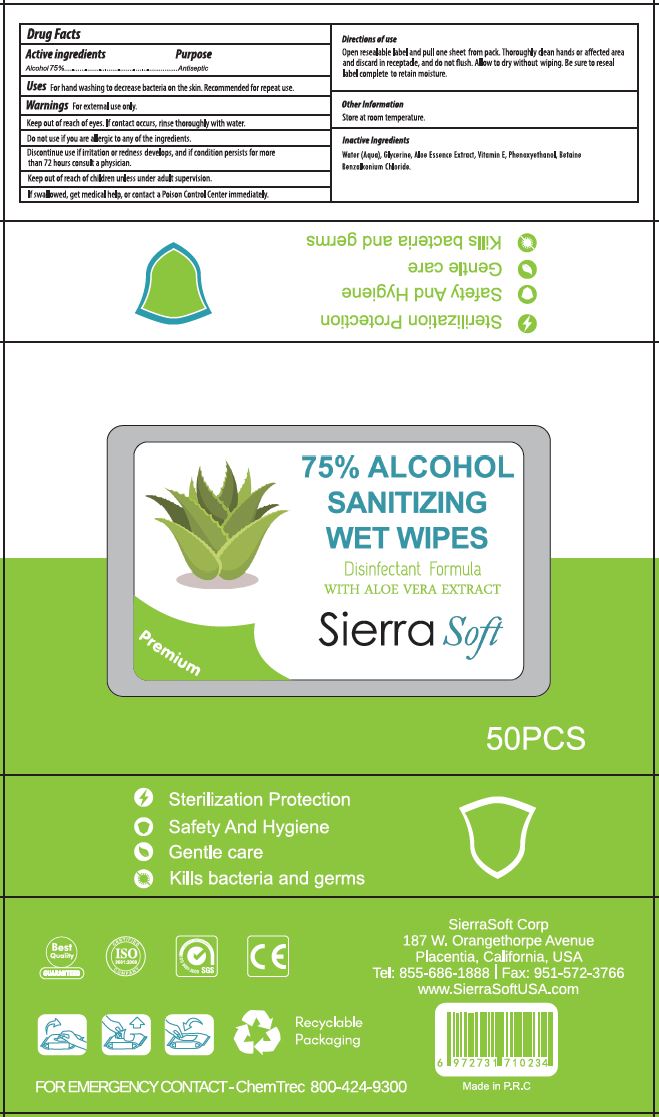
CWGC (as PLD) - SIERRA SOFT SANITIZING WET WIPES 75% ETHYL ALCOHOL (70415-109) - DELIST
SANITIZING WET WIPES by
Drug Labeling and Warnings
SANITIZING WET WIPES by is a Otc medication manufactured, distributed, or labeled by CWGC LA Inc., Fujian Yifa Healthcare Products Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANITIZING WET WIPES 75 PERCENT- ethyl alcohol solution
CWGC LA Inc.
----------
CWGC (as PLD) - SIERRA SOFT SANITIZING WET WIPES 75% ETHYL ALCOHOL (70415-109) - DELIST
Warnings
FOR EXTERNAL USE ONLY.
KEEP OUT OF REACH OF EYES. IF CONTACT OCCURS, RINSE THOROUGHLY WITH WATER.
DO NOT USE IF YOU ARE ALLERGIC TO ANY OF THE INGREDIENTS.
DISCONTINUE USE IF IRRITATION OR REDNESS DEVELOPS, AND IF CONDITION PERSISTS FOR MORE THAN 72 HOURS CONSULT A PHYSICIAN.
KEEP OUT OF REACH OF CHILDREN UNLESS UNDER ADULT SUPERVISION.
IF SWALLOWED, GET MEDICAL HELP, OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Directions
OPEN RESEALABLE LABEL AND PULL ONE SHEET FROM PACK. THOROUGHLY CLEAN HANDS OR AFFECTED AREA AND DISCARD IN RECEPTACLE, AND DO NOT FLUSH. ALLOW TO DRY WITHOUT WIPING. BE SURE TO RESEAL LABEL COMPLETE TO RETAIN MOISTURE.
| SANITIZING WET WIPES
75 PERCENT
ethyl alcohol solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CWGC LA Inc. (034967904) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.