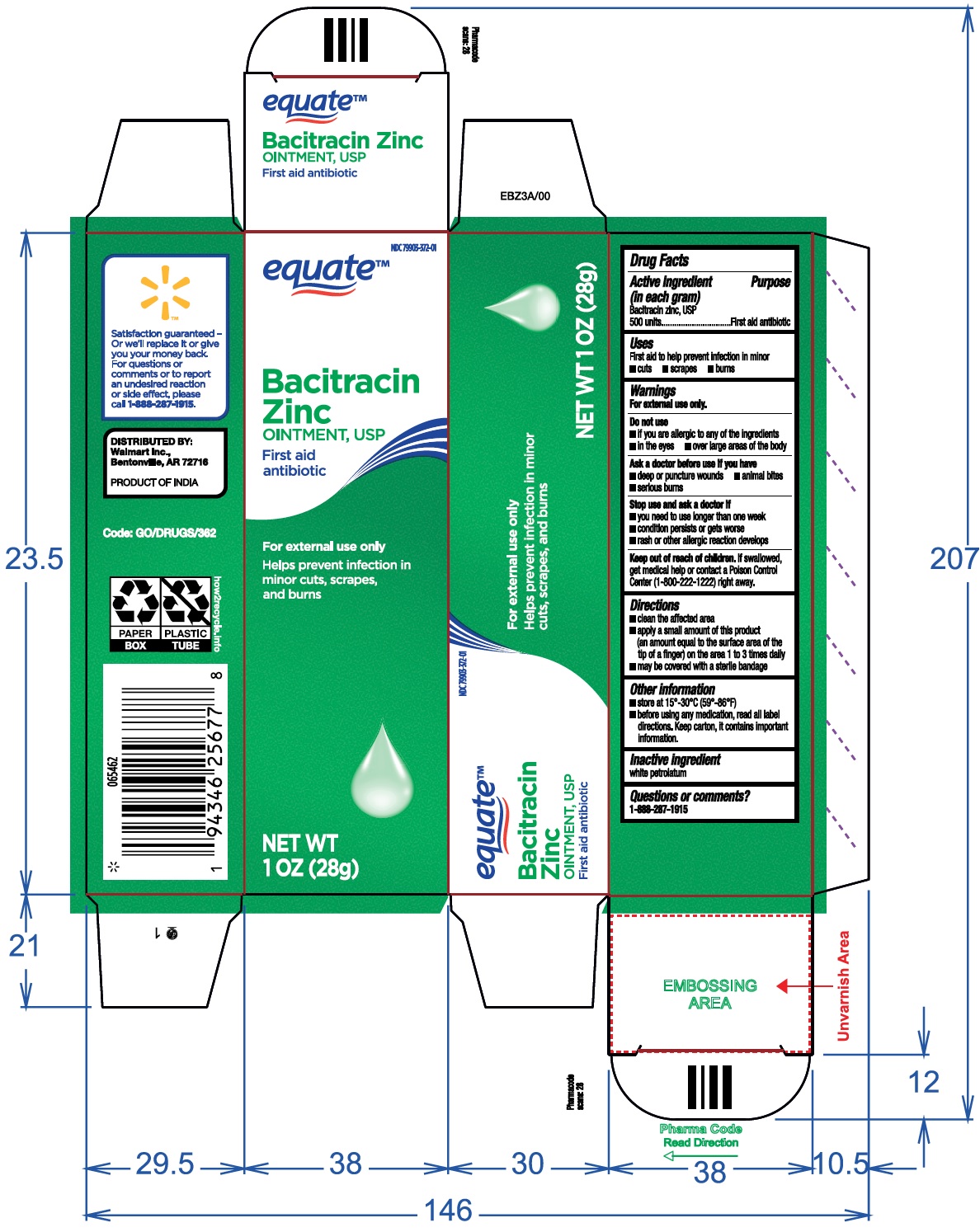
BACITRACIN ZINC OINTMENT, USP First aid antibiotic
BACITRACIN ZINC by
Drug Labeling and Warnings
BACITRACIN ZINC by is a Otc medication manufactured, distributed, or labeled by Walmart Inc., Encube Ethicals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BACITRACIN ZINC - first aid antibiotic ointment ointment
Walmart Inc.
----------
BACITRACIN ZINC OINTMENT, USP
First aid antibiotic
ACTIVE INGREDIENT(S)
(in each gram)
Bacitracin zinc, USP 500 units ............. First aid antibiotic
STOP USE AND ASK DOCTOR IF
- you need to use longer than one week
- condition persist or gets worse
- rash or other allergic reaction develops
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
DIRECTIONS
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
OTHER INFORMATION
- store at 150-300C(590-860F)
- before using any medication, read all label directions. Keep carton, it contains important information.
| BACITRACIN ZINC
first aid antibiotic ointment ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Walmart Inc. (051957769) |
| Registrant - Encube Ethicals Private Limited (915834105) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Encube Ethicals Private Limited | 725076298 | ANALYSIS(79903-375) , LABEL(79903-375) , MANUFACTURE(79903-375) , PACK(79903-375) | |
Revised: 1/2025
<
Document Id: dc5c4c5f-908d-48ab-a317-4310f9a9ba3a
Set id: 1226bf97-622b-423d-91c6-634f31dd4fb2
Version: 3
Effective Time: 20250131
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.