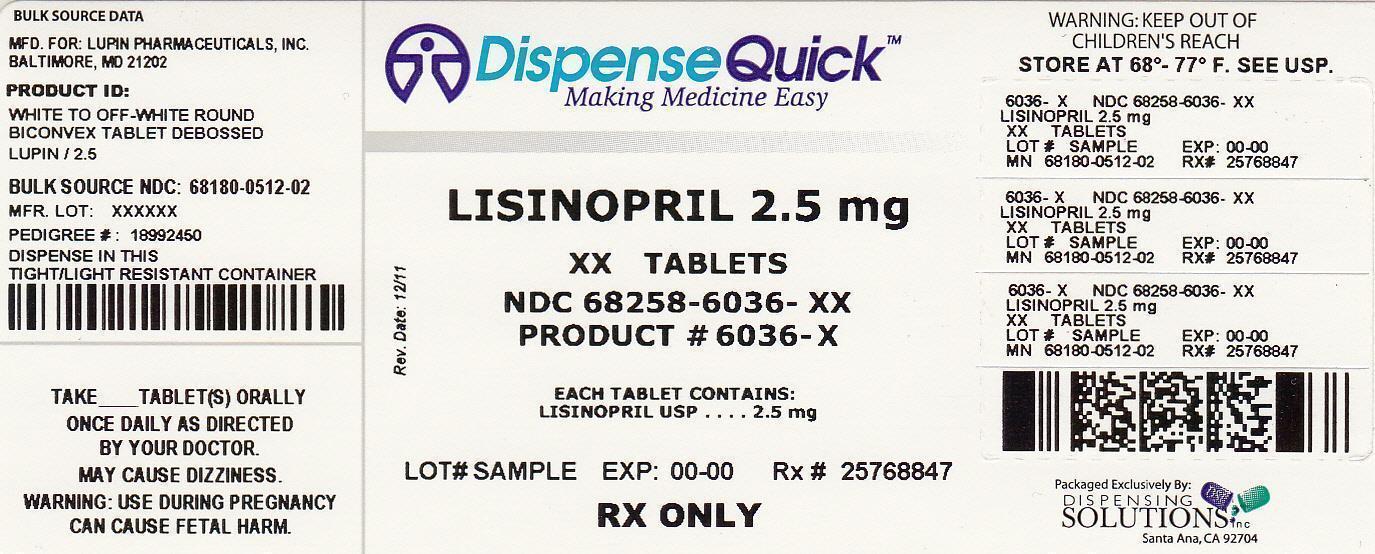
Lisinopril by Dispensing Solutions, Inc. / PSS World Medical, Inc. LISINOPRIL tablet
Lisinopril by
Drug Labeling and Warnings
Lisinopril by is a Prescription medication manufactured, distributed, or labeled by Dispensing Solutions, Inc., PSS World Medical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, lisinopril should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
-
DESCRIPTION
Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5(2H2O and its structural formula is:
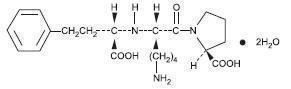
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.53. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol.
Lisinopril tablets are supplied as 2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg and 40 mg tablets for oral administration.
Inactive Ingredients:
2.5 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, starch.
5 mg, 10 mg, 20 mg and 30 mg tablets – colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, red iron oxide, starch.
40 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, starch, yellow iron oxide.
- CONTRAINDICATIONS
-
ADVERSE REACTIONS
Lisinopril has been found to be generally well tolerated in controlled clinical trials involving 1969 patients with hypertension or heart failure. For the most part, adverse experiences were mild and transient.
Hypertension
In clinical trials in patients with hypertension treated with lisinopril, discontinuation of therapy due to clinical adverse experiences occurred in 5.7% of patients. The overall frequency of adverse experiences could not be related to total daily dosage within the recommended therapeutic dosage range.
For adverse experiences occurring in greater than 1% of patients with hypertension treated with lisinopril or lisinopril plus hydrochlorothiazide in controlled clinical trials, and more frequently with lisinopril and/or lisinopril plus hydrochlorothiazide than placebo, comparative incidence data are listed in the table below:
PERCENT OF PATIENTS IN CONTROLLED STUDIES Lisinopril(n=1349)Incidence(discontinuation) Lisinopril/ Hydrochlorothiazide(n=629)Incidence(discontinuation) Placebo(n=207)Incidence(discontinuation) Body as a Whole Fatigue 2.5 (0.3) 4.0 (0.5) 1.0 (0.0) Asthenia 1.3 (0.5) 2.1 (0.2) 1.0 (0.0) Orthostatic Effects 1.2 (0.0) 3.5 (0.2) 1.0 (0.0) Cardiovascular Hypotension 1.2 (0.5) 1.6 (0.5) 0.5 (0.5) Digestive Diarrhea 2.7 (0.2) 2.7 (0.3) 2.4 (0.0) Nausea 2.0 (0.4) 2.5 (0.2) 2.4 (0.0) Vomiting 1.1 (0.2) 1.4 (0.1) 0.5 (0.0) Dyspepsia 0.9 (0.0) 1.9 (0.0) 0.0 (0.0) Musculoskeletal Muscle Cramps 0.5 (0.0) 2.9 (0.8) 0.5 (0.0) Nervous/Psychiatric Headache 5.7 (0.2) 4.5 (0.5) 1.9 (0.0) Dizziness 5.4 (0.4) 9.2 (1.0) 1.9 (0.0) Paresthesia 0.8 (0.1) 2.1 (0.2) 0.0 (0.0) Decreased Libido 0.4 (0.1) 1.3 (0.1) 0.0 (0.0) Vertigo 0.2 (0.1) 1.1 (0.2) 0.0 (0.0) Respiratory Cough 3.5 (0.7) 4.6 (0.8) 1.0 (0.0) Upper Respiratory Infection 2.1 (0.1) 2.7 (0.1) 0.0 (0.0) Common Cold 1.1 (0.1) 1.3 (0.1) 0.0 (0.0) Nasal Congestion 0.4 (0.1) 1.3 (0.1) 0.0 (0.0) Influenza 0.3 (0.1) 1.1 (0.1) 0.0 (0.0) Skin Rash 1.3 (0.4) 1.6 (0.2) 0.5 (0.5) Urogenital Impotence 1.0 (0.4) 1.6 (0.5) 0.0 (0.0) Chest pain and back pain were also seen, but were more common on placebo than lisinopril.
Heart Failure
In patients with heart failure treated with lisinopril for up to four years, discontinuation of therapy due to clinical adverse experiences occurred in 11% of patients. In controlled studies in patients with heart failure, therapy was discontinued in 8.1% of patients treated with lisinopril for 12 weeks, compared to 7.7% of patients treated with placebo for 12 weeks.
The following table lists those adverse experiences which occurred in greater than 1% of patients with heart failure treated with lisinopril or placebo for up to 12 weeks in controlled clinical trials, and more frequently on lisinopril than placebo.Controlled Trials Controlled Trials Lisinopril (n=407)
Incidence
(discontinuation)
12 weeksPlacebo (n=155)
Incidence
(discontinuation)
12 weeksBody as a Whole Chest Pain 3.4 (0.2) 1.3 (0.0) Abdominal Pain 2.2 (0.7) 1.9 (0.0) Cardiovascular Hypotension 4.4 (1.7) 0.6 (0.6) Digestive Diarrhea 3.7 (0.5) 1.9 (0.0) Nervous/Psychiatric Dizziness 11.8 (1.2) 4.5 (1.3) Headache 4.4 (0.2) 3.9 (0.0) Respiratory Upper Respiratory Infection 1.5 (0.0) 1.3 (0.0) Skin Rash 1.7 (0.5) 0.6 (0.6) Also observed at > 1% with lisinopril but more frequent or as frequent on placebo than lisinopril in controlled trials were asthenia, angina pectoris, nausea, dyspnea, cough, and pruritus.
Worsening of heart failure, anorexia, increased salivation, muscle cramps, back pain, myalgia, depression, chest sound abnormalities, and pulmonary edema were also seen in controlled clinical trials, but were more common on placebo than lisinopril.
In the two-dose ATLAS trial in heart failure patients, withdrawals due to adverse events were not different between the low and high groups, either in total number of discontinuation (17-18%) or in rare specific events (less than 1%). The following adverse events, mostly related to ACE inhibition, were reported more commonly in the high dose group:
% of patients Events High Dose
(N=1568)Low Dose
(N=1596)Dizziness 18.9 12.1 Hypotension 10.8 6.7 Creatinine-increased 9.9 7.0 Hyperkalemia 6.4 3.5 NPN* increased 9.2 6.5 Syncope 7.0 5.1
*NPN = non-protein nitrogen
Acute Myocardial Infarction
In the GISSI-3 trial, in patients treated with lisinopril for six weeks following acute myocardial infarction, discontinuation of therapy occurred in 17.6% of patients.
Patients treated with lisinopril had a significantly higher incidence of hypotension and renal dysfunction compared with patients not taking lisinopril.
In the GISSI-3 trial, hypotension (9.7%), renal dysfunction (2%), cough (0.5%), post infarction angina (0.3%), skin rash and generalized edema (0.01%), and angioedema (0.01%) resulted in withdrawal of treatment. In elderly patients treated with lisinopril, discontinuation due to renal dysfunction was 4.2%.
Other clinical adverse experiences occurring in 0.3% to 1.0% of patients with hypertension or heart failure treated with lisinopril in controlled clinical trials and rarer, serious, possibly drug-related events reported in uncontrolled studies or marketing experience are listed below, and within each category are in order of decreasing severity:
Body as a Whole
Anaphylactoid reactions (see WARNINGS, Anaphylactoid and Possibly Related Reactions ), syncope, orthostatic effects, chest discomfort, pain, pelvic pain, flank pain, edema, facial edema, virus infection, fever, chills, malaise.
Cardiovascular: Cardiac arrest; myocardial infarction or cerebrovascular accident possibly secondary to excessive hypotension in high risk patients (see WARNINGS, Hypotension ); pulmonary embolism and infarction, arrhythmias (including ventricular tachycardia, atrial tachycardia, atrial fibrillation, bradycardia and premature ventricular contractions), palpitations, transient ischemic attacks, paroxysmal nocturnal dyspnea, orthostatic hypotension, decreased blood pressure, peripheral edema, vasculitis.
Digestive: Pancreatitis, hepatitis (hepatocellular or cholestatic jaundice) (see WARNINGS, Hepatic Failure ), vomiting, gastritis, dyspepsia, heartburn, gastrointestinal cramps, constipation, flatulence, dry mouth.
Hematologic: Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia and thrombocytopenia.
Endocrine: Diabetes mellitus, inappropriate antidiuretic hormone secretion.
Metabolic: Weight loss, dehydration, fluid overload, gout, weight gain. Cases of hypoglycemia in diabetic patients on oral antidiabetic agents or insulin have been reported in post-marketing experience (See PRECAUTIONS, Drug Interactions ).
Musculoskeletal: Arthritis, arthralgia, neck pain, hip pain, low back pain, joint pain, leg pain, knee pain, shoulder pain, arm pain, lumbago.
Nervous System/Psychiatric: Stroke, ataxia, memory impairment, tremor, peripheral neuropathy (e.g., dysesthesia), spasm, paresthesia, confusion, insomnia, somnolence, hypersomnia, irritability, nervousness and mood alterations (including depressive symptoms).
Respiratory System: Malignant lung neoplasms, hemoptysis, pulmonary infiltrates, bronchospasm, asthma, pleural effusion, pneumonia, eosinophilic pneumonitis, bronchitis, wheezing, orthopnea, painful respiration, epistaxis, laryngitis, sinusitis, pharyngeal pain, pharyngitis, rhinitis, rhinorrhea.
Skin: Urticaria, alopecia, herpes zoster, photosensitivity, skin lesions, skin infections, pemphigus, erythema, flushing, diaphoresis, cutaneous pseudolymphoma. Other severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens-Johnson syndrome; causal relationship has not been established.
Special Senses: Visual loss, diplopia, blurred vision, tinnitus, photophobia, taste disturbances.
Urogenital System: Acute renal failure, oliguria, anuria, uremia, progressive azotemia, renal dysfunction (see PRECAUTIONS and DOSAGE AND ADMINISTRATION ), pyelonephritis, dysuria, urinary tract infection, breast pain.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia and leukocytosis. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Angioedema: Angioedema has been reported in patients receiving lisinopril (0.1%) with an incidence higher in Black than in non-Black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with lisinopril should be discontinued and appropriate therapy instituted immediately (See WARNINGS).
In rare cases, intestinal angioedema has been reported in post marketing experience.
Hypotension: In hypertensive patients, hypotension occurred in 1.2% and syncope occurred in 0.1% of patients with an incidence higher in Black than in non-Black patients. Hypotension or syncope was a cause of discontinuation of therapy in 0.5% of hypertensive patients. In patients with heart failure, hypotension occurred in 5.3% and syncope occurred in 1.8% of patients. These adverse experiences were possibly dose-related (see above data from ATLAS Trial) and caused discontinuation of therapy in 1.8% of these patients in the symptomatic trials. In patients treated with lisinopril for six weeks after acute myocardial infarction, hypotension (systolic blood pressure (100 mmHg) resulted in discontinuation of therapy in 9.7% of the patients. (See WARNINGS).
Fetal/Neonatal Morbidity and Mortality: See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Cough: See PRECAUTIONS, Cough
Pediatric Patients: No relevant differences between the adverse experience profile for pediatric patients and that previously reported for adult patients were identified.
CLINICAL LABORATORY TEST FINDINGS
Serum Electrolytes: Hyperkalemia (See PRECAUTIONS ), hyponatremia.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 2% of patients with essential hypertension treated with lisinopril alone. Increases were more common in patients receiving concomitant diuretics and in patients with renal artery stenosis (See PRECAUTIONS). Reversible minor increases in blood urea nitrogen and serum creatinine were observed in approximately 11.6% of patients with heart failure on concomitant diuretic therapy. Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.4 g% and 1.3 vol%, respectively) occurred frequently in patients treated with lisinopril but were rarely of clinical importance in patients without some other cause of anemia. In clinical trials, less than 0.1% of patients discontinued therapy due to anemia. Hemolytic anemia has been reported; a causal relationship to lisinopril cannot be excluded.
Liver Function Tests: Rarely, elevations of liver enzymes and/or serum bilirubin have occurred (See WARNINGS, Hepatic Failure).
In hypertensive patients, 2.0% discontinued therapy due to laboratory adverse experiences, principally elevations in blood urea nitrogen (0.6%), serum creatinine (0.5%) and serum potassium (0.4%).
In the heart failure trials, 3.4% of patients discontinued therapy due to laboratory adverse experiences; 1.8% due to elevations in blood urea nitrogen and/or creatinine and 0.6% due to elevations in serum potassium.
In the myocardial infarction trial, 2.0% of patients receiving lisinopril discontinued therapy due to renal dysfunction (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration); less than 1.0% of patients discontinued therapy due to other laboratory adverse experiences: 0.1% with hyperkalemia and less than 0.1% with hepatic enzyme alterations.
-
OVERDOSAGE
Following a single oral dose of 20 g/kg no lethality occurred in rats, and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis (See WARNINGS, Anaphylactoid Reactions During Membrane Exposure).
-
HOW SUPPLIED
Lisinopril Tablets, USP are available as:
2.5 mg tablet is a white to off-white, round, biconvex uncoated tablet with ‘LUPIN" debossed on one side and "2.5" on other side. They are available as follows:
Bottles of 90 NDC: 68180-512-09
Bottles of 100 NDC: 68180-512-01
Bottles of 500 NDC: 68180-512-02
Bottles of 1000 NDC: 68180-512-03
5 mg tablet is a pink coloured, round, biconvex uncoated tablet with "5" debossed on one side and breakline on other side. They are available as follows:
Bottles of 90 NDC: 68180-513-09
Bottles of 100 NDC: 68180-513-01
Bottles of 500 NDC: 68180-513-02
Bottles of 1000 NDC: 68180-513-03
Bottles of 5000 NDC: 68180-513-05
10 mg tablet is a pink coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "10" on other side. They are available as follows:
Bottles of 90 NDC: 68180-514-09
Bottles of 100 NDC: 68180-514-01
Bottles of 500 NDC: 68180-514-02
Bottles of 1000 NDC: 68180-514-03
Bottles of 5000 NDC: 68180-514-05
20 mg tablet is a pink coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "20" on other side. They are available as follows:
Bottles of 90 NDC: 68180-515-09
Bottles of 100 NDC: 68180-515-01
Bottles of 500 NDC: 68180-515-02
Bottles of 1000 NDC: 68180-515-03
Bottles of 5000 NDC: 68180-515-05
30 mg tablet is a red coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "30" on other side. They are available as follows:
Bottles of 90 NDC: 68180-516-09
Bottles of 100 NDC: 68180-516-01
Bottles of 500 NDC: 68180-516-02
Bottles of 1000 NDC: 68180-516-03
40 mg tablet is a yellow coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "40" on other side. They are available as follows:
Bottles of 90 NDC: 68180-517-09
Bottles of 100 NDC: 68180-517-01
Bottles of 500 NDC: 68180-517-02
Bottles of 1000 NDC: 68180-517-03
Bottles of 2000 NDC: 68180-517-04
Storage:
Store at 20º to 25ºC (68º to 77ºF)[see USP Controlled Room Temperature]. Protect from moisture, freezing and excessive heat. Dispense in a tight container.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LISINOPRIL
lisinopril tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68258-6036(NDC:68180-512) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LISINOPRIL (UNII: E7199S1YWR) (LISINOPRIL ANHYDROUS - UNII:7Q3P4BS2FD) LISINOPRIL 2.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MANNITOL (UNII: 3OWL53L36A) COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 7mm Flavor Imprint Code LUPIN;2;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68258-6036-3 30 in 1 BOTTLE 2 NDC: 68258-6036-9 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077321 11/17/2005 Labeler - Dispensing Solutions, Inc. (066070785) Registrant - PSS World Medical, Inc. (101822682) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 relabel(68258-6036) , repack(68258-6036)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.